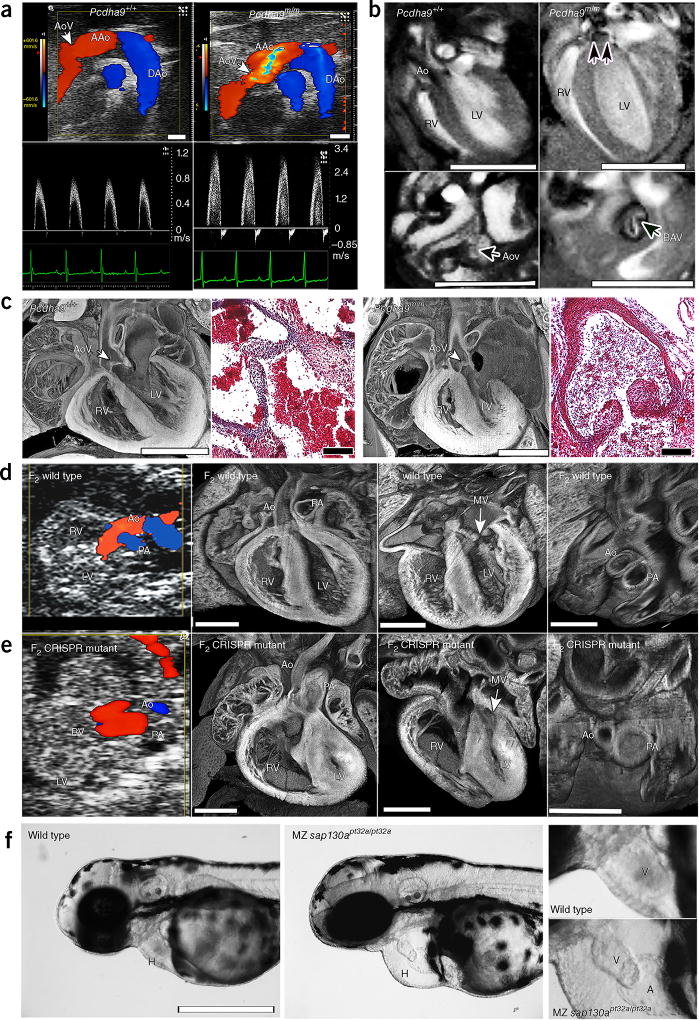
Figure 2.
Phenotypes of Pcdha9m/m mice and CRISPR–Cas9-generated Sap130; Pcdha9 mice and sap130a-mutant zebrafish. (a–c) Ohia Pcdha9m/m mutants exhibit aortic valve defects. (a) Echocardiography of adult Pcdha9m/m and wild-type mice. Color-flow (top) and spectral Doppler (bottom) imaging, showing a high-velocity jet (>3 m/s) across the aortic valve, thus indicating aortic stenosis (supplementary Video 5). (b) Functional magnetic resonance imaging (MRI) showing the bicuspid aortic valve. MRI of Pcdham/m mouse from a, showing BAV with doming (double arrows) during systole (supplementary Videos 6 and 7). LV hypertrophy, as determined by M-mode imaging (LV anterior wall/posterior wall: 0.45/0.64 mm in control versus 0.91/0.91 mm in mutant). (c) Histopathology of newborn Pcdha9m/m versus wild-type mice. Compared with wild type, the mutant mouse showed immature thickened aortic valves with increased trichrome staining, thus indicating abnormal matrix deposition. (d,e) CRISPR–Cas9-targeted Sap130; Pcdha9 mutants exhibit HLHS. The CRISPR-generated F2Sap130m/m; Pcdha9m/m mutant showed a small LV with a small flow stream from the ascending aorta. Aortic and pulmonary blood flow originated from the RV, thus indicating a double-outlet RV variant of HLHS (supplementary Video 8), as confirmed by histopathology showing a small LV with hypoplastic aorta and MV (supplementary Video 9). (f) CRISPR–Cas9-targeted sap130a zebrafish exhibiting a small ventricle. Maternal–zygotic (MZ) sap130apt32a/pt32a mutants, compared with wild type, exhibited hearts (H) with small ventricles and pericardial effusion. An enlarged view of the ventricle (V) is shown at right. AAo, ascending aorta; DAo, descending aorta; AoV, aortic valve; LV, left ventricle; RV, right ventricle; Ao, aorta; MV, mitral valve; PA, pulmonary artery; H, heart; V, ventricle; A, atrium. Scale bars: b, 5 mm; a,c (histopathology), 1 mm; c (trichrome stain), 0.1 mm; (d–f) 0.5 mm.