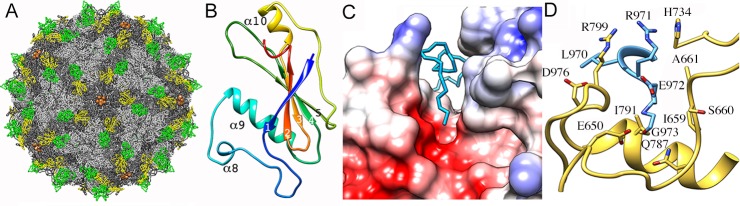
Fig 5. Putative enzyme activity on the RnQV1 outer capsid surface.
(A) The P2 Asp227-Tyr350 domain (yellow) and P4 Val648-His805 domain (green) of a heterodimer are shown on the outer RnQV1 surface. P4 plugs at the five-fold axes are shown (orange). (B) The P2 Asp227-Tyr350 domain, rainbow-colored, with the topology found on the capsid surface. The five anti-parallel β-strands are indicated (1–5), as are the three helices (α8, α9, α10). (C) The P4 surface cleft (shown with electrostatic potentials) in which the P2 C terminus is located (blue, sticks). (D) Close-up of the P2 C-terminal segment (blue) in the P4 surface cavity (yellow), oriented as in c. Residues are displayed as sticks.