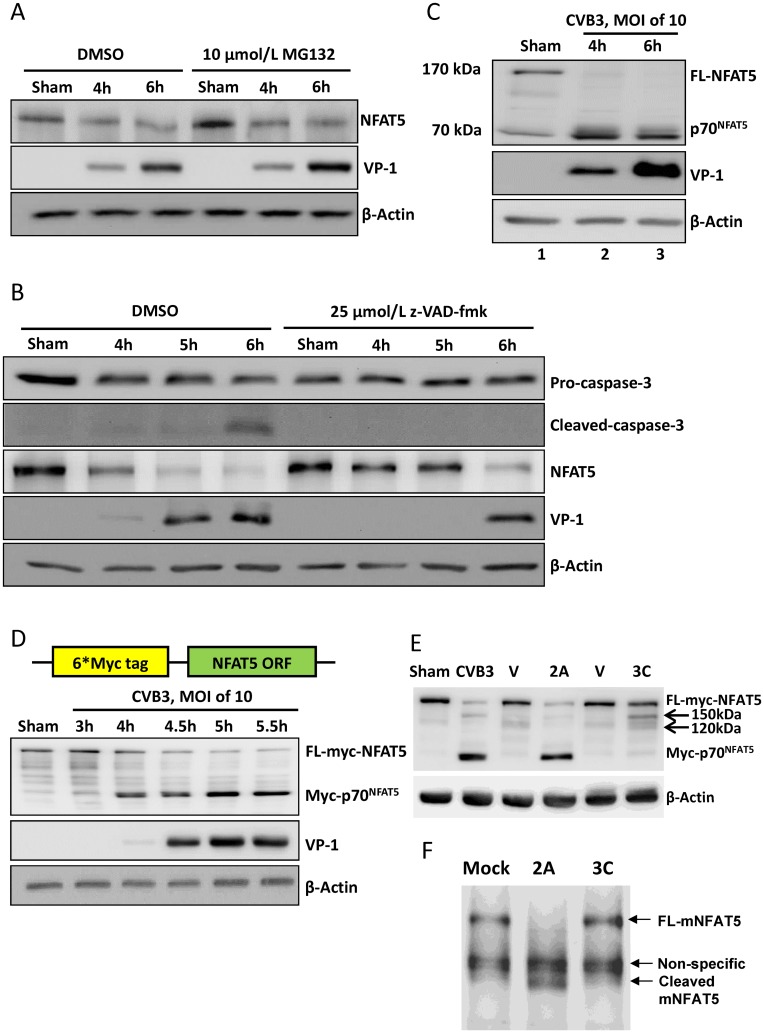
Fig 2. NFAT5 is cleaved by viral proteases 2A and 3C.
HeLa cells were treated with 10 μM MG132 (A) or 25 μM z-VAD-fmk (B) and then infected with CVB3 at an MOI of 10. At indicated time points pi, the cellular proteins were subjected to Western blot analysis of NFAT5 and other proteins using the indicated antibodies. β-actin was used as a loading control. (C) HeLa cells were infected by CVB3 at an MOI of 10 for 4 and 6 h and then subjected to Western blot analysis using an antibody against the N-terminal epitope of NFAT5. (D) HeLa cells transfected with a plasmid expressing the 6*myc-NFAT5 fusion protein (upper panel) were infected with CVB3 or sham-infected as described above and subjected to Western blot analysis using an antibody against myc tag (lower panel). (E) HeLa cells expressing myc-NFAT5 were transfected with pIRES-2A (2A), pIRES-3C (3C) or vector only (V). At 36 h pt, the cells were subjected to Western blot analysis using an antibody against myc tag. The cells infected with CVB3 or sham-infected with PBS were used as controls. Arrows indicate the 3C cleavage bands. (F) Tissue homogenate from mouse heart was incubated with recombinant 2A or 3C for 8 h. Then the mouse NFAT5 (mNFAT5) N-terminal epitope was detected by Western blot using a specific antibody.