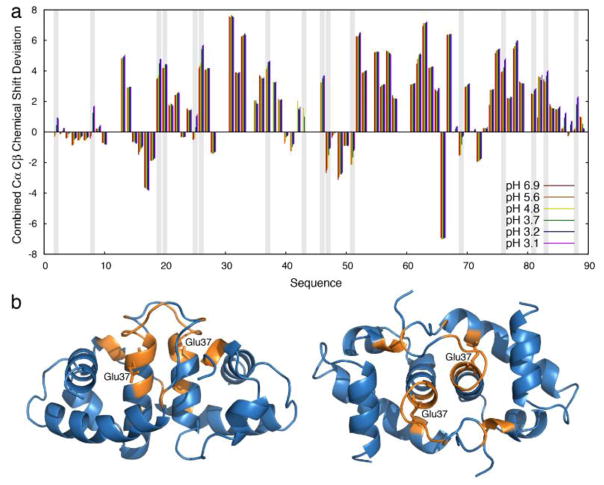
Fig. 3.
NMR investigation of HdeA secondary structure upon pH variation. (a) Residue specific combined Cα and Cβ chemical shift deviations from their respective random coil values (positive values indicates α-helices, negative values indicate β-sheets) at pHs ranging from 6.9 (red) to 3.1 (purple). Vertical grey bars indicate Asp or Glu that can be protonated in this pH range. Apart from these residues, combined Cα and Cβ chemical shift deviations show only minor changes, suggesting that the previously observed increased H/D exchange upon lowering the pH is mainly due to a non-directly visible intermediate. (b) Residues disappearing (no longer observable) upon pH increase from 5.6 to 6.9 are shown in orange on the HdeA dimeric structure (left, right: two different views) (PDB ID: 1BG8) [20]. The affected residues cluster closely around Glu37 (side-chain is labeled), suggesting a key role for this residue in HdeA stabilization at mildly acidic pH.