Fig. 4.
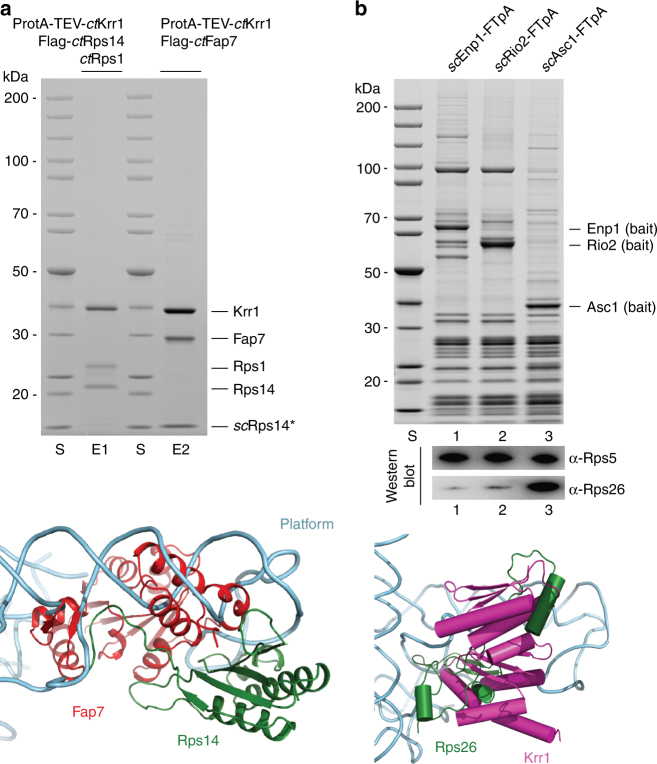
Interaction network of Krr1 on the 90S pre-ribosome. a ctKrr1 forms a complex with ribosomal proteins Rps14 and Rps1. The indicated heterotrimeric ctKrr1-ctRps14-ctRps1 and hybrid complex ctKrr1-ctFap7-scRps14, which includes the endogenous yeast ribosomal protein Rps14, were assembled by co-expression of the indicated Chaetomium thermophilum factors in yeast followed by split-tag tandem affinity-purification. The reconstituted complexes were analyzed via SDS-PAGE (4–12%) and Coomassie staining. The labeled protein bands were identified by mass spectrometry. The depicted crystal structure of Fap7 (red) bound to Rps14 (green) (lower panel) shows how Fap7 binding clashes with binding of Rps14 to rRNA helix 23 (light blue) within the 90S pre-ribosome. Superimposition was carried out based on the Rps14 proteins from both the isolated platform of the 90S pre-ribosome (PDB: 5JPQ) and the crystal structure of the Rps14-Fap7 complex (PDB: 4CW7). For better visualization, only the crystal structure of Rps14-Fap7 and the rRNA of the platform were shown in cartoon representation. b Binding of ribosomal protein Rps26 clashes with the Krr1 binding site on the 90S particle. Tandem affinity-purification of FTpA-tagged Enp1, Rio2 and Asc1. Eluates were TCA-precipitated and analyzed by SDS-PAGE (4–12%) and Coomassie staining (upper panel) or western blotting (lower panel) using indicated antibodies. The lower panel shows a superimposition of Rps14 proteins from both the isolated platform of the 90S pre-ribosome (PDB:5JPQ) and the crystal structure of the mature 40S (PDB: 4V5O). For a better visualization, only Rps26 (green), Krr1 (pink) and the rRNA (blue) of the 40S platform were depicted in the cartoon representation. The experiments were performed at least twice with consistent results. Uncropped images are shown in Supplementary Fig. 9. S molecular weight protein standard, E1 and E2 eluates 1 and 2