Fig. 1.
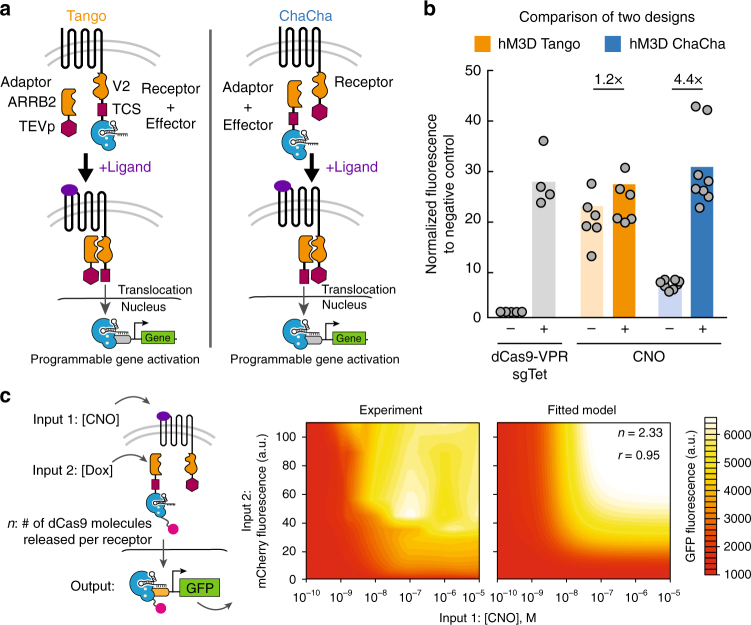
The GPCR ChaCha design outperforms the Tango design. a Two design schemes of coupling CRISPR-Cas9 function to the activity of GPCR. Left: the Tango design fused the effector protein (dCas9-VPR as shown) to the C-terminus of GPCR via a V2 tail sequence and a TEV cleavage sequence (TCS). An adaptor protein, Beta-Arrestin 2 (ARRB2), is fused to the Tobacco Etch Virus protease (TEVp). Right: in the ChaCha design, we fused the effector to the adaptor protein via the TCS, and fused the TEVp to the receptor via the V2 tail. Upon ligand binding to the receptor, both systems recruit the adaptor to the V2 tail, and the protease specifically cleaves at the TCS, releasing the effector protein that translocates into the nucleus for sgRNA-directed gene regulation. b Comparison of the performance of Tango (orange) and ChaCha (blue) designs using the synthetically evolved GPCR, hM3D in HEK293T cells with or without 20 μM clozapine-N-oxide (CNO), the ligand for hM3D (see Methods section for detailed experimental procedure). The free dCas9-VPR (gray) with and without a targeting sgRNA (sgTet) are used as positive and negative controls. The data were normalized to the free dCas9-VPR without a targeting sgRNA. The data represent two independent experiments with 4–8 technical replicates, and the bars represent the mean. c Estimation of the number of dCas9 molecules (n) released per receptor in the ChaCha design. Left, schematic of a stable cell line containing hM3D-CRISPR ChaCha with Doxycycline (Dox), inducing the expression of the ARRB2-TCS-dCas9-VPR and CNO inducing the receptor activity. Middle, GFP activation levels after 3 days of Dox induction and CNO treatment. Right, GFP activation levels based on the rate-model fit at steady state (r = 0.95); n is a measure of dCas9 molecules released per receptor (see Methods section for the detailed model)