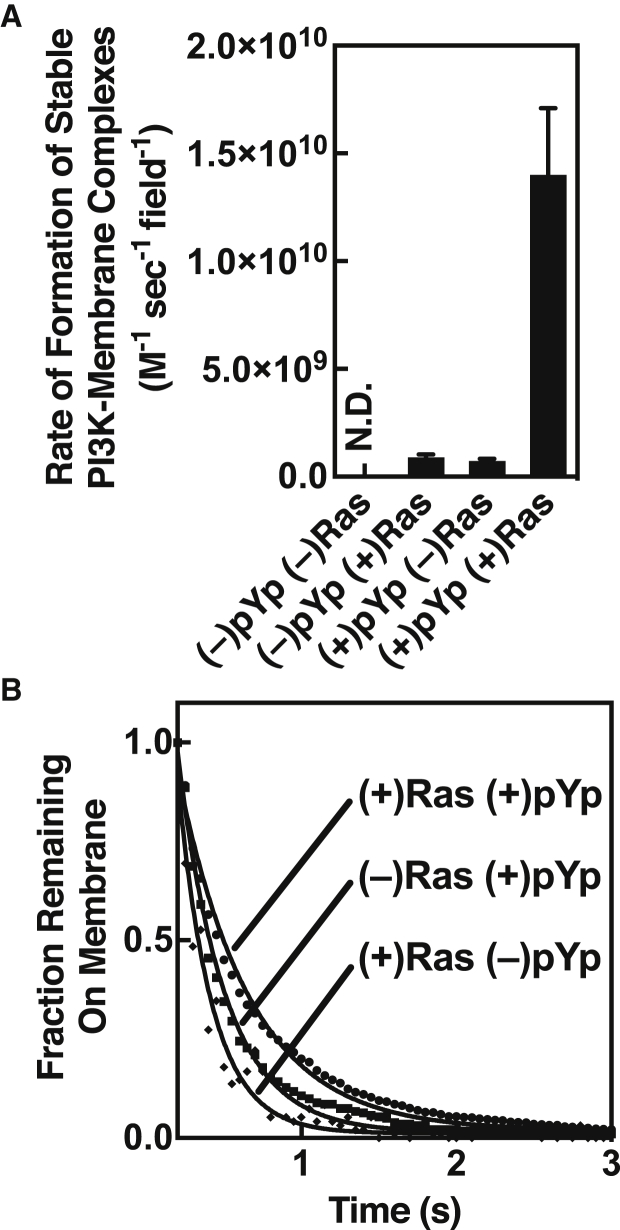
Figure 4.
Effect of pYp and H-Ras on PI3Kα association-dissociation kinetics. (A) Relative appearance rates of stable, fluor-PI3Kα single-particle diffusion tracks were measured under different activating conditions. These appearance rates were used to calculate the formation rate of active, stably bound PI3Kα complexes on the membrane surface as follows. The number of PI3Kα tracks with bound-state lifetimes exceeding 100 ms in a TIRFM movie was determined, then divided by movie time and bulk PI3Kα concentration to yield an operationally defined appearance rate. Each rate was generated from groups of three replicate movies acquired on each of five different days (n = 15). (B) Off rates of fluor-PI3Kα from the membrane surface, calculated by binning the bound-state lifetimes of stable PI3Kα single-particle diffusion tracks at least 150 ms in length and fitting with a single-exponential decay. Each decay curve was generated from groups of three replicate movies acquired on each of five different days (n = 15). The resulting exponential decay constants were: 2.9 ± 0.6 s−1 for (–)pYp (+)Ras, 2.5 ± 0.6 s−1 for (+)pYp (–)Ras, and 1.9 ± 0.2 s−1 for (+)pYp (+)Ras. No kinetic data could be obtained for (–)pYp (–)Ras due to its very low density on the bilayer surface. In all cases, in both (A) and (B), H-Ras is loaded with non-hydrolyzable GTP analog GMPPNP.