Table 2.
Primary efficacy endpoint results: ASAS20 response rate at week 12
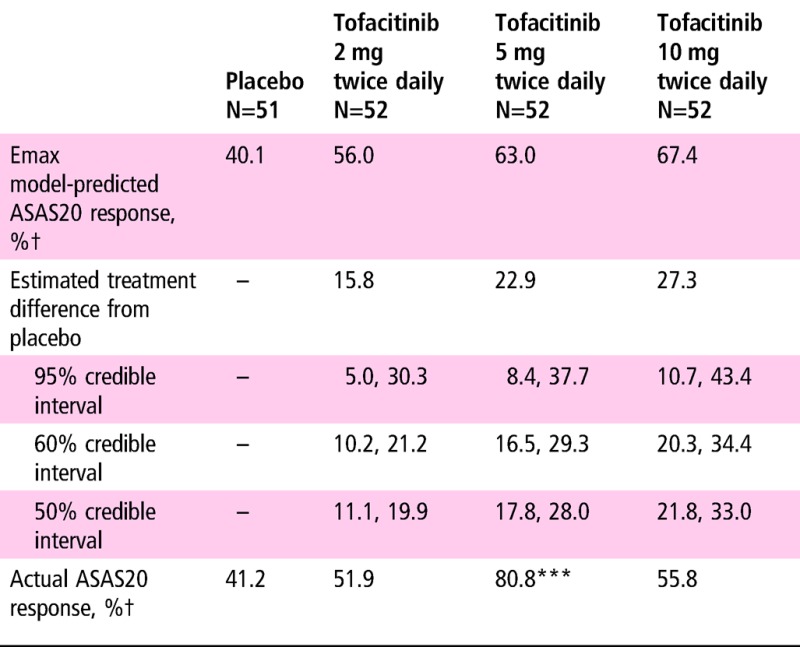
|
***p≤0.001 versus placebo by normal approximation.
†Non-responder imputation was used: ASAS20 value was set to be non-responsive for patients who had no ASAS20 component data.
ASAS, Assessment of SpondyloArthritis International Society.
