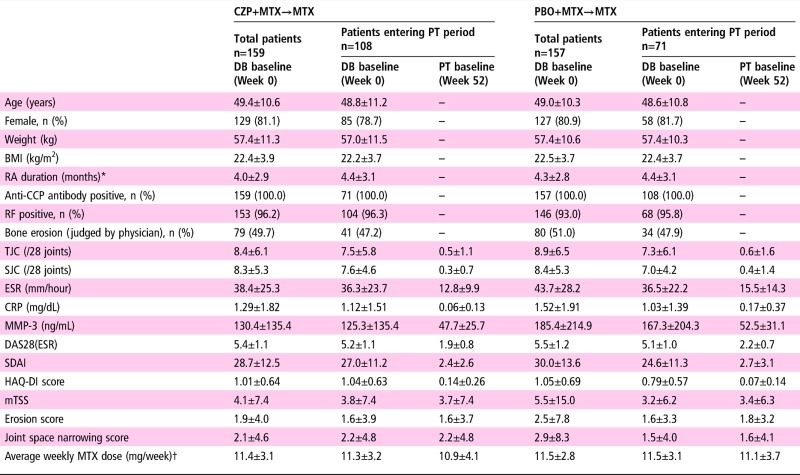
Table 1.
Baseline demographics and patient characteristics
|
Values are mean±SD unless otherwise indicated. Data in DB baseline columns represent average during weeks 0–104, whereas data in PT baseline columns represent average during weeks 52–104.
*Time from onset of persistent arthritic symptoms. †MTX dose was initiated at 8 mg/week and escalated to the maximum tolerated dose (up to 16 mg/week) by week 8.
BMI, body mass index; CCP, cyclic citrullinated peptide; CRP, C reactive protein; CZP, certolizumab pegol; DB, double blind; ESR, erythrocyte sedimentation rate; HAQ-DI, Health Assessment Questionnaire Disability Index; MMP-3, matrix metalloproteinase-3; mTSS, modified total Sharp score; MTX, methotrexate; PBO, placebo; PT, post treatment; RA, rheumatoid arthritis; RF, rheumatoid factor; SJC, swollen joint count; TJC, tender joint count.
