Abstract
Despite widespread use of glyburide to treat pregnancy-related hyperglycemia, the dosing regimen is based in large part on pharmacokinetic and pharmacodynamic studies in men and nonpregnant women. Like many medications used by pregnant women, adequate pharmacokinetic and pharmacodynamic data in pregnancy have been sorely lacking. This lack of information can lead to both overdosing with excessive side effects and under-dosing with an inadequate therapeutic response. Both of these problems may apply to glyburide use in pregnancy. This commentary provides a pharmacologic basis for altering the glyburide administration regimen. Taking glyburide 1 hour before a meal may improve efficacy in patients with pregnancy-related hyperglycemia.
BACKGROUND
The Obstetric-Fetal Pharmacology Research Unit Network was established by the Eunice Kennedy Shriver National Institute of Child Health and Human Development in 2005 to expand research on pharmacologic agents used by pregnant women. We have previously reported on a multicenter study of glyburide pharmacokinetics and pharmacodynamics in pregnant women with gestational diabetes mellitus (GDM).1 The application of the pharmacologic data obtained from that study to clinical issues affecting management of women with GDM receiving or about to receive glyburide therapy is the focus of the current report. The primary findings of our previous investigation are summarized below.
After oral ingestion of glyburide, concentrations of the drug began to increase within 30–60 minutes, peaked in 2–3 hours, and returned to baseline by 8 hours. Glucose, insulin, and C-peptide increased shortly after meal ingestion.
The apparent oral clearance of glyburide from the plasma of pregnant women after oral administration was approximately double the rate of oral clearance in nonpregnant women.
Glyburide crossed the placental barrier with a cord-to-maternal plasma concentration ratio of 0.7.
In this article, we discuss how these findings may affect the use of glyburide in women with GDM.
FINDINGS AND CLINICAL APPLICATION
Plasma Concentration Versus Time Characteristics of Oral Glyburide, Insulin, C-Peptide, and Glucose
The pharmacokinetic parameters and metabolic effects of glyburide and other sulfonylureas have been well characterized in men and nonpregnant patients with type 2 diabetes.2,3 Additional insight has been gleaned from animal and in vitro studies. Collectively, these studies have demonstrated that in nonpregnant women, glyburide peaks 4 hours after ingestion and plasma concentrations decrease over the ensuing 8 hours and the drug is still detectable 24 hours after ingestion.2,3 Glyburide sensitizes the pancreas to enhance insulin secretion particularly after a meal or a glucose load.4,5 The insulin response to meals or a glucose stimulus is enhanced when glyburide is given before a meal.4 In patients with type 2 diabetes mellitus receiving glyburide orally without a meal, insulin secretion is not increased nor is fasting insulin concentration.4,6 Glucose concentrations decrease with glyburide administration even in the fasting state suggesting that at least some of the glucose-lowering effect of glyburide occurs through noninsulin-mediated mechanisms.6 In addition, in humans, two of the metabolites of glyburide exhibit glucose-lowering activity, which appears to be insulin-mediated.7 There is also evidence that the response to glyburide in non-pregnant patients does not increase at concentrations above 10 mg daily.8 Furthermore, the response to glyburide and other sulfonureas may be blunted with higher or constantly elevated concentrations.9 Thus, downregulation of the response to glyburide may be seen with higher drug concentrations. These observations support the concept of using the lowest effective dose to reduce the amount of metabolite and to reduce the degree of desensitization.
There are very limited data regarding the pharmacodynamic and metabolic effects of glyburide in pregnant women. Data from a subset of the cohort recruited in our previous Obstetric-Fetal Pharmacology Research Unit trial are depicted in Figures 1 and 2.1 Figure 1 demonstrates that the drug is more rapidly eliminated in pregnancy than in the nonpregnant state leading to markedly lower plasma concentrations in pregnant women.1 Figure 2 depicts the change in concentrations of glucose, C-peptide, and insulin after a standardized mixed meal in women who were given glyburide 1 hour before the meal. On average, plasma glyburide concentrations in pregnant women with GDM do not increase until 1 hour after drug ingestion, peak at 2–3 hours, and return to baseline by 8 hours. Glucose and insulin increase shortly after ingestion of the meal and C-peptide also begins to increase after the meal. This pharmacokinetic and metabolic profile suggests that the currently recommended glyburide administration regimen may not be optimal for pregnant women. The glyburide administration regimen currently in use suggests that treatment should be initiated with once-daily dosing administered with breakfast or the first main meal. Additionally, once blood sugar is controlled, the usual maintenance dose ranges between 1.25 mg and 20 mg daily, which may be given as a single dose or in divided doses.2 Studies of women with GDM generally used once- or twice-daily dosage with the medication given in the morning and often at the start of a meal or with no mention of timing related to a meal.10,11 This dosing regimen may contribute to hypoglycemia or, conversely, to a perceived lack of glucose control. First, based on the results depicted in Figure 2, glyburide should be administered 30–60 minutes before a meal so that the rise in glyburide concentration precedes the elevation in blood sugar seen with a meal. This would likely improve efficacy by optimizing the insulin response to a meal because insulin secretion with a meal or glucose elevations is proportional to the glyburide concentration.4,8
Fig. 1.
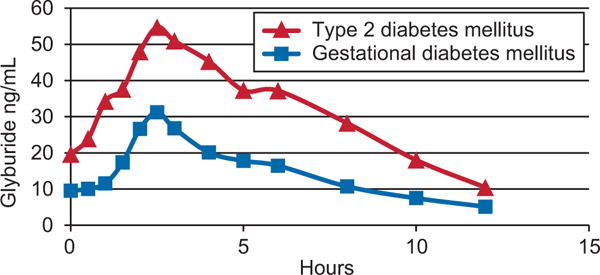
Plasma concentration of glyburide after a 2.5-mg oral dose in women with type 2 diabetes (n=8) or gestational diabetes (n=18). The data in this figure are taken from a subset of the data from the Obstetric-Fetal Pharmacology Research Unit collected, as described in Hebert MF, Ma X, Naraharisetti SB, Krudys KM, Umans JG, Hankins GD, et al, for the Obstetric-Fetal Pharmacology Research Unit Network. Are we optimizing gestational diabetes treatment with glyburide? The pharmacologic basis for better clinical practice. Clin Pharmacol Ther 2009;85:607–14.1
Caritis. Glyburide Use in Women With GDM. Obstet Gynecol 2013.
Fig. 2.
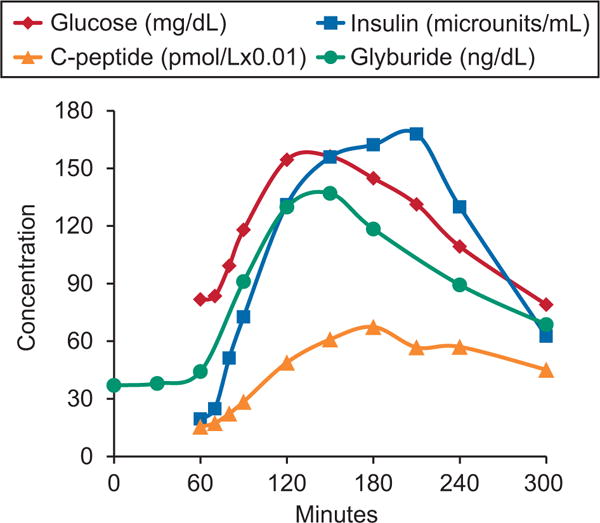
Average plasma concentrations of glyburide (ng/dL), glucose (mg/dL), insulin (microunits/mL), and C-peptide (pmol/L×0.01) in 40 women with gestational diabetes after a mixed meal tolerance test.1 Glyburide concentrations are dose normalized to a 1.25-mg dose twice daily. Glyburide was administered at time 0 and the mixed meal load at 60 minutes. Concentrations of C-peptide and glyburide are scaled to enable better visualization of change over time course on a single graph. The data in this figure are taken from a subset of the data the Obstetric-Fetal Pharmacology Research Unit collected, as described in Hebert MF, Ma X, Naraharisetti SB, Krudys KM, Umans JG, Hankins GD, et al, for the Obstetric-Fetal Pharmacology Research Unit Network. Are we optimizing gestational diabetes treatment with glyburide? The pharmacologic basis for better clinical practice. Clin Pharmacol Ther 2009;85:607–14.1
Caritis. Glyburide Use in Women With GDM. Obstet Gynecol 2013.
Second, because glyburide concentrations return to baseline within 8–10 hours of ingestion with daytime dosing (Fig. 1), the insulin response to a dinnertime meal will be reduced if the glyburide was taken only in the morning. Thus, a predinner dose of glyburide may be needed. Attempts to control postdinner hyperglycemia with a morning dose of glyburide would require higher morning doses, which may result in postbreakfast insulin-related hypoglycemia or later-day hypoglycemia mediated by glyburide metabolites or other noninsulin-related mechanisms. Thus, a morning, dinner, and, in some women, a bedtime dose might be required.
Another issue to consider regarding the concentration versus time in Figure 2 is the timing of the postmeal glucometer testing as it relates to the timing of the glyburide dose. The usual glucometer testing times are 1 or 2 hours after start of a meal. Ideally the increase in insulin secretion should coincide with the time of maximal glucose excursion. This, as depicted in Figure 2, occurs when glyburide is administered 1 hour before a meal. In this scenario, glucometer testing whether performed 1 or 2 hours after start of a meal will accurately reflect the effect of the given glyburide dose. If blood glucose exceeds the 140 or 120 mg/dL threshold at 1 or 2 hours, respectively, the dose of glyburide would be appropriately increased. If, on the other hand, glyburide is given with the start of the meal and glucometer testing is performed at 1 or 2 hours after the start of the meal, the full effect of the given glyburide dose is not achieved at the time of glucometer testing and the glucose values either at 1 or 2 hours after a meal may exceed the 140- or 120-mg/dL threshold resulting in a potentially avoidable dosage increase. This scenario may be responsible for some of the hypoglycemia seen with glyburide. Clearly additional studies are required to provide clarity on this issue and to validate the recommendations proposed here.
Increased Plasma Glyburide Clearance in Pregnancy
The increased clearance of glyburide in pregnancy means that to achieve a plasma concentration in pregnant women comparable to the concentration achieved in nonpregnant women, more than twice the dose is needed (Fig. 1). This difference is not surprising because similar changes in dosage requirements for pregnant women are seen with other medications. Glyburide is metabolized primarily by the cytochrome P450 enzymes (CYP2C9, CYP3A, and CYP2C19). Two of these enzymes (CYP2C9 and CYP3A) demonstrate increased activity in pregnancy and may account for the more rapid apparent oral clearance of glyburide in pregnancy. Glyburide is eliminated from plasma by conversion to one or more metabolic products, which are eliminated in the feces or urine. Very little unmetabolized glyburide is found in the urine so the increased apparent oral clearance of the drug cannot be attributed to the increase in renal blood flow seen in pregnancy.
This finding of more rapid apparent oral clearance of glyburide in pregnancy raises the question of whether the maximal dose of glyburide should be increased to twice the current recommended dose for nonpregnant women and men, ie, 40 mg daily rather than 20 mg daily. Clinical studies of glyburide suggest that in 5–20% of gestational diabetes cases, acceptable blood sugar control is not achieved. It could be argued that the failure rate could be reduced if a higher dose was used. This seems pharmacologically reasonable but several factors require consideration before such an approach is undertaken. First, there is evidence that the effect of glyburide on meal-related insulin secretion is achieved with doses of 10 mg/d in nonpregnant women and higher doses do not seem to enhance that effect.8 Second, the amount of glyburide metabolites with glucose-lowering properties will be increased with higher doses of glyburide. These metabolites may be responsible for some of the hypoglycemic episodes seen with glyburide. Consequently, keeping the concentration of these metabolites low appears potentially advantageous. The prevalence of maternal hypoglycemia with glyburide therapy varies from 0% to 20%. Doubling the currently recommended maximal dose may increase that risk. The incidence of inadequate control as well as the incidence of maternal hypoglycemia, however, may be reduced by changing the timing of glyburide administration (see previous section, “Plasma Concentration Compared With Time Characteristics of Oral Glyburide, Insulin, C-Peptide, and Glucose”).
Finally, in considering a dosage increase of glyburide during pregnancy, there is the issue of fetal and neonatal side effects. This is discussed subsequently (“Placental Transport of Glyburide”). The incidence of neonatal hypoglycemia, which is reported to be approximately 20%, may be increased with a higher glyburide dosage, especially because glyburide does cross the placenta. Additionally, any potential long-term fetal and neonatal effects beyond those ascertainable in the nursery (such as fetal programming effects) may be affected with a higher maternal dose. Clearly, more research is required to address the question of whether a higher dosage of glyburide can or should be used in pregnancy.
Placental Transport of Glyburide
We have reported that glyburide crosses the placenta with average cord-to-maternal plasma concentration ratio of 0.7.1 This relationship persisted over various time intervals from the last maternal dose. These findings differ from those previously published.10 These differences, however, can be attributed to assay methodology. We used high-performance liquid chromatography with mass spectrometry assay with a limit of quantification of 0.25 ng/mL, which is much more sensitive than the previously used assay of high-performance liquid chromatography with ultraviolet detection whose limit of detection is 10 ng/mL.
The implications of placental transfer of glyburide are substantial. Until the work of Langer et al,10 the use of oral hypoglycemic agents such as glyburide and metformin had been limited because of concern for fetal exposure to the medication. With the report of Langer et al10 that glyburide was not detectable in cord blood and the in vitro evidence that glyburide was actively transported from the fetal side of the placenta to the mother, glyburide use increased, particularly after the drug was shown to be comparable to insulin in controlling maternal blood sugar and reducing the incidence of macrosomia. With the demonstration that glyburide is detectable in fetal blood, the risk–benefit equation requires reassessment. To date, however, the neonatal experience with maternal glyburide doses up to 20 mg/d has been favorable.11 The rate of neonatal hypoglycemia is approximately 20% whether the mother is receiving glyburide or insulin. The rates of neonatal complications, biometric measurements, skinfold thickness, body fat, and cord insulin concentrations are similar in neonates whose mothers received insulin or glyburide.11 Although the placenta possesses efflux transporters that pump glyburide from the fetus to the mother and the fetus possesses the metabolic enzymes required for glyburide metabolism, the fetal drug concentrations are a bit lower but still reflective of maternal concentrations.1 The reassuring information on short-term neonatal outcomes must be balanced by the potential for adverse long-term outcomes either from poor glucose control or glyburide exposure.
If the dose of glyburide were to be doubled so that maternal drug concentrations in women with GDM are comparable to drug concentrations recommended for nonpregnant women or men, the incidence of neonatal hypoglycemia may be increased because glyburide reaches the fetus freely. Clearly, more research is required to address the question of whether a higher dosage of glyburide can or should be used in pregnancy.
SUMMARY
Based on our findings, we suggest that glyburide should be administered 30–60 minutes before a meal. In some patients, a dose of glyburide may be required before dinner. Additional research is required to determine whether higher dosages can or should be used in pregnant women with GDM. Glyburide crosses the placenta and this should be factored into the risk–benefit equation when considering glyburide or insulin use in women with GDM.
Acknowledgments
Supported by grants from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (HD047905 and HD047892). The content is solely the responsibility of the authors and does not necessarily represent the official views of the Eunice Kennedy Shriver National Institute of Child Health and Human Development or the National Institutes of Health.
The authors thank Shannon Clark, MD, David M. Haas, MD, MS, Gary Hankins, MD, Zhaoxia Ren, MD, PhD, Raman Venkataramanan, PhD, and Anne Zajicek, MD, PharmD, from the Obstetric-Fetal Pharmacology Research Unit Network for reviewing and making suggestions for improving the manuscript.
Footnotes
Financial Disclosure
The authors did not report any potential conflicts of interest.
References
- 1.Hebert MF, Ma X, Naraharisetti SB, Krudys KM, Umans JG, Hankins GD, et al. for the Obstetric-Fetal Pharmacology Research Unit Network Are we optimizing gestational diabetes treatment with glyburide? the pharmacologic basis for better clinical practice. Clin Pharmacol Ther. 2009;85:607–14. doi: 10.1038/clpt.2009.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pfizer. Available at: http://www.pfizer.com/files/products/uspi_micronase.pdf. Retrieved April 1, 2013.
- 3.Rendell M. The role of sulphonylureas in the management of type 2 diabetes mellitus. Drugs. 2004;64:1339–58. doi: 10.2165/00003495-200464120-00006. [DOI] [PubMed] [Google Scholar]
- 4.Sartor G, Lundquist I, Melander A, Scherstén B, Wåhlin-Boll E. Improved effect of glibenclamide on administration before breakfast. Eur J Clin Pharmacol. 1982;21:403–8. doi: 10.1007/BF00542327. [DOI] [PubMed] [Google Scholar]
- 5.Sartor G, Melander A, Scherstén B, Wåhlin-Boll E. Serum glibenclamide in diabetic patients, and influence of food on the kinetics and effects of glibenclamide. Diabetologia. 1980;18:17–22. doi: 10.1007/BF01228296. [DOI] [PubMed] [Google Scholar]
- 6.Go EH, Kyriakidou-Himonas M, Berelowitz M. Effects of glipizide GITS and glibenclamide on metabolic control, hepatic glucose production, and insulin secretion in patients with type 2 diabetes. Diabetes Metab Res Rev. 2004;20:225–31. doi: 10.1002/dmrr.443. [DOI] [PubMed] [Google Scholar]
- 7.Rydberg T, Jönsson A, Røder M, Melander A. Hypoglycemic activity of glyburide (glibenclamide) metabolites in humans. Diabetes Care. 1994;17:1026–30. doi: 10.2337/diacare.17.9.1026. [DOI] [PubMed] [Google Scholar]
- 8.Groop L, Wåhlin-Boll E, Groop PH, Tötterman KJ, Melander A, Tolppanen EM, et al. Pharmacokinetics and metabolic effects of glibenclamide and glipizide in type 2 diabetics. Eur J Clin Pharmacol. 1985;28:697–704. doi: 10.1007/BF00607919. [DOI] [PubMed] [Google Scholar]
- 9.Melander A, Donnelly R, Rydberg T. Is there a concentration–effect relationship for sulphonylureas? Clin Pharmacokinet. 1998;34:181–8. doi: 10.2165/00003088-199834030-00001. [DOI] [PubMed] [Google Scholar]
- 10.Langer O, Conway DL, Berkus MD, Xenakis EM, Gonzales O. A comparison of glyburide and insulin in women with gestational diabetes mellitus. N Engl J Med. 2000;343:1134–8. doi: 10.1056/NEJM200010193431601. [DOI] [PubMed] [Google Scholar]
- 11.Nicholson W, Baptiste-Roberts K. Oral hypoglycaemic agents during pregnancy: the evidence for effectiveness and safety. Best Pract Res Clin Obstet Gynaecol. 2011;25:51–63. doi: 10.1016/j.bpobgyn.2010.10.018. [DOI] [PubMed] [Google Scholar]


