Fig. 2.
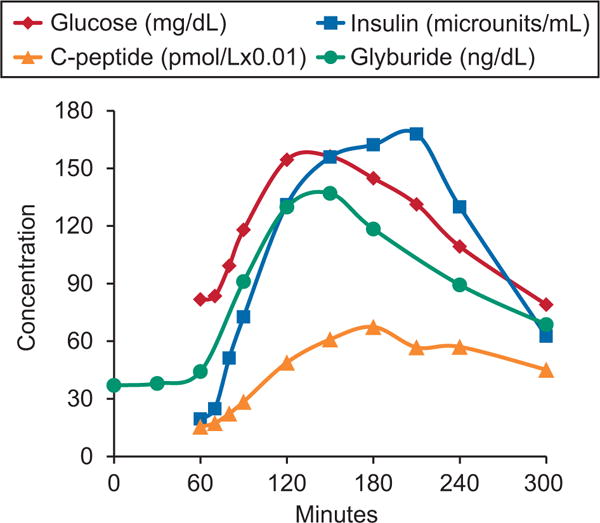
Average plasma concentrations of glyburide (ng/dL), glucose (mg/dL), insulin (microunits/mL), and C-peptide (pmol/L×0.01) in 40 women with gestational diabetes after a mixed meal tolerance test.1 Glyburide concentrations are dose normalized to a 1.25-mg dose twice daily. Glyburide was administered at time 0 and the mixed meal load at 60 minutes. Concentrations of C-peptide and glyburide are scaled to enable better visualization of change over time course on a single graph. The data in this figure are taken from a subset of the data the Obstetric-Fetal Pharmacology Research Unit collected, as described in Hebert MF, Ma X, Naraharisetti SB, Krudys KM, Umans JG, Hankins GD, et al, for the Obstetric-Fetal Pharmacology Research Unit Network. Are we optimizing gestational diabetes treatment with glyburide? The pharmacologic basis for better clinical practice. Clin Pharmacol Ther 2009;85:607–14.1
Caritis. Glyburide Use in Women With GDM. Obstet Gynecol 2013.
