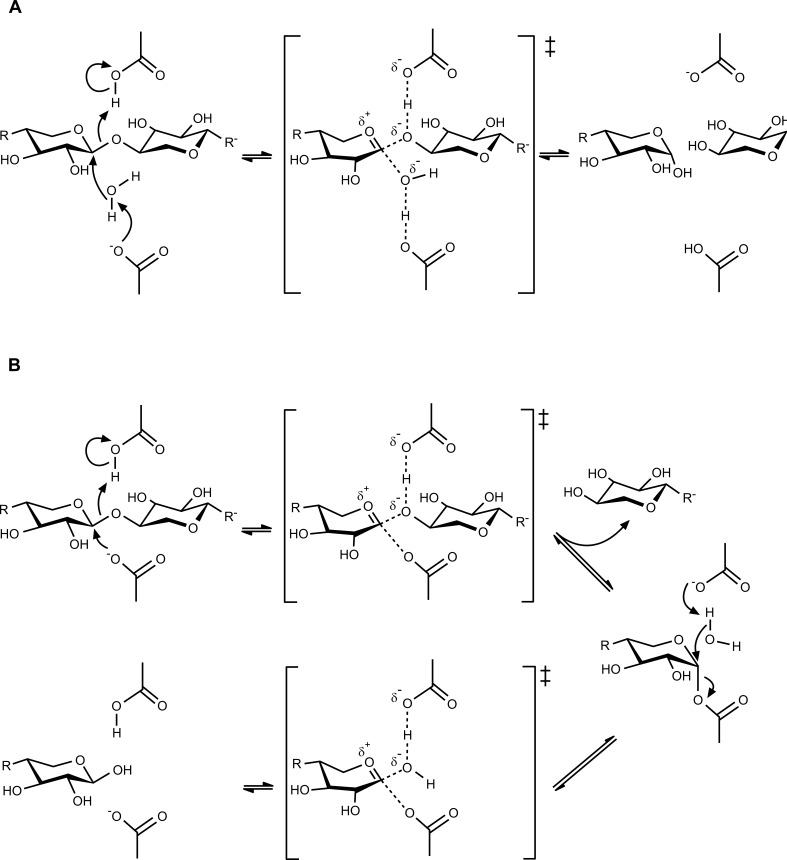
Fig. (1).
Representation of the chemical mechanisms for endo-xylanases based on the general mechanism of glycoside hydrolases [7]. (A) Inverting. The reaction occurs via single displacement where one carboxylic group is the general base and the other one is the general acid, and these residues are situated around 10.5 Å from each other. (B) Retaining. The reaction occurs via double displacement where one carboxylic group acts as general acid/base, and the other one as nucleophile forming a covalent intermediate. In this case, both carboxylic groups are situated around 5.5 Å from each other. In both mechanisms the transition states adopt distorted conformations due the oxocarbenium-ion-like structure, with boat (2,5B in GH11) and half-chair (4H3 in GH10) conformations being most energetically favored [8].