FIG 3.
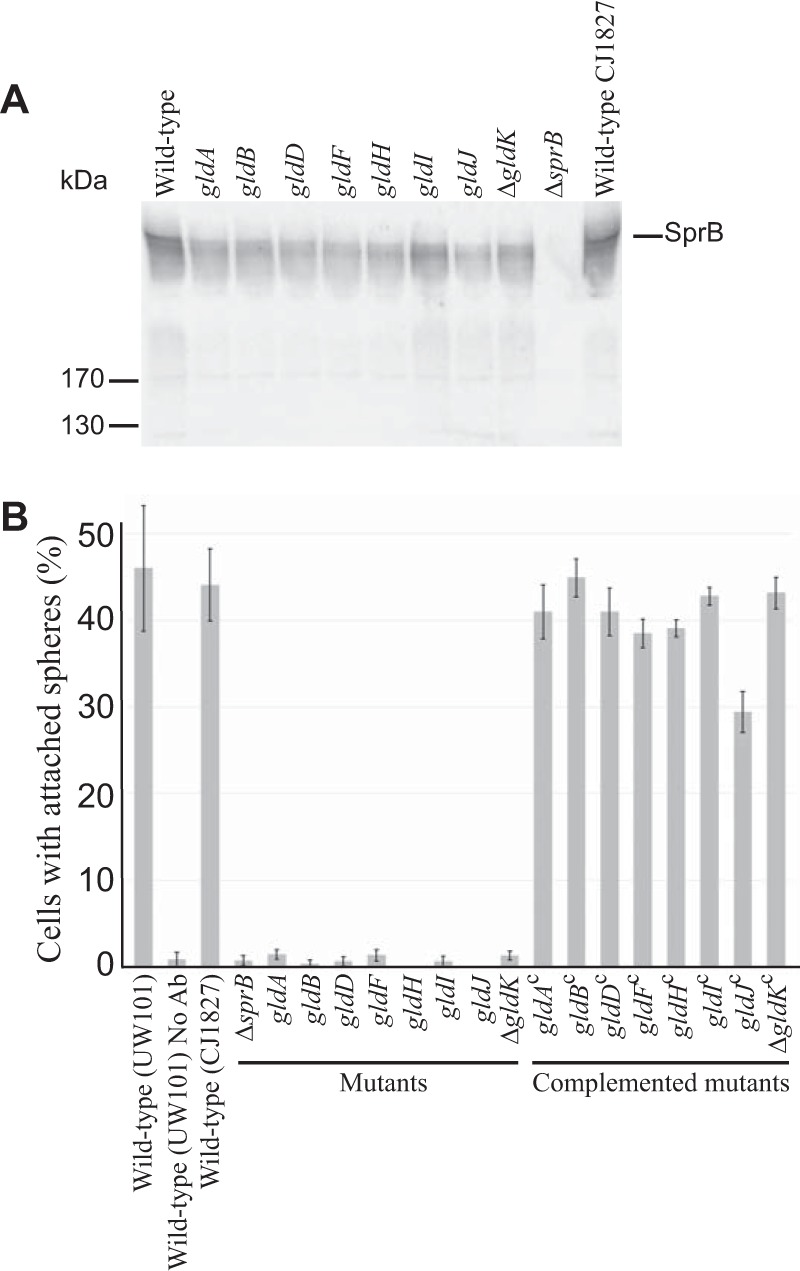
Cells with mutations in gldA, gldB, gldD, gldF, gldH, gldI, and gldJ produce the motility adhesin SprB but fail to secrete it to the cell surface. (A) Immunodetection of SprB in cells of wild-type or mutant F. johnsoniae strains. Whole cells were analyzed from cultures of wild-type F. johnsoniae UW101, streptomycin-resistant “wild-type” CJ1827, gldA mutant UW101-288, gldB mutant CJ569, gldD mutant CJ282, gldF mutant CJ787, gldH mutant CJ1043, gldI mutant UW102-41, gldJ mutant UW102-80, ΔgldK mutant CJ2122, and ΔsprB mutant CJ1922. Cell samples corresponded to 15 μg of protein per lane. Samples were separated by SDS-PAGE, and SprB was detected using antiserum against SprB. SprB is predicted to be 669 kDa after the removal of its N-terminal signal peptide, and it has previously been shown to migrate as a diffuse band (13). (B) Detection of SprB on the surface of wild-type or mutant cells. Anti-SprB antiserum and 0.5-μm-diameter protein G-coated polystyrene spheres were added to cells and examined using a phase-contrast microscope, as described in Materials and Methods. Images were recorded for 30 s, and 100 randomly selected cells were examined for the presence of spheres that remained attached to the cells during this time. Wild-type and mutant strains were as described in panel A. “No Ab” indicates no antisera were added to this sample. Complemented mutants (indicated by superscript “c”) carried the appropriate plasmids to express GldA (pSA21), GldB (pDH223), GldD (pMM209), GldF and GldG (pMK314), GldH (pMM293), GldI (pMM291), GldJ (pMM313), and GldK (pTB99). Error bars indicate standard deviations from three measurements.
