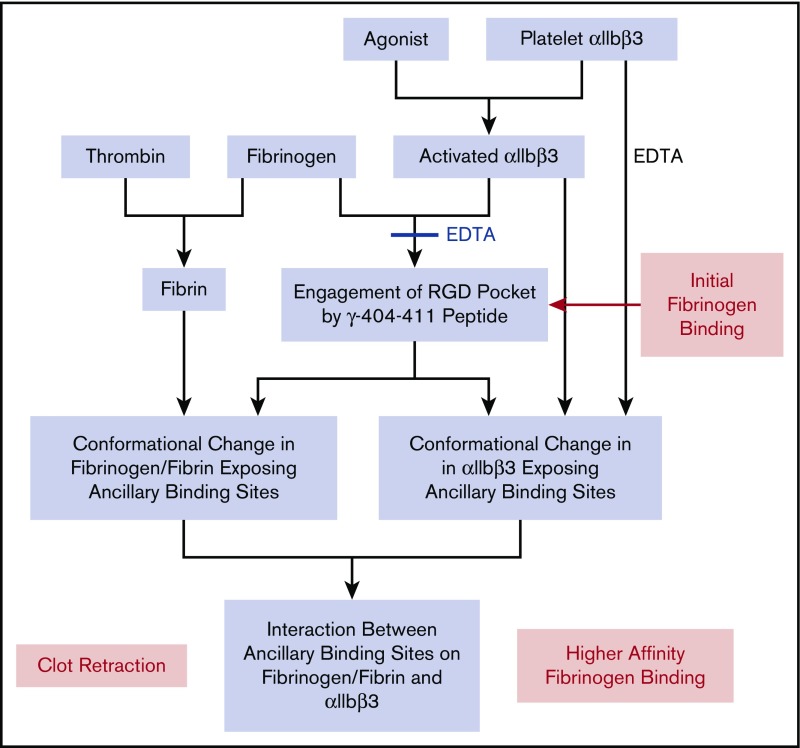
Figure 7.
Working model of the multistep process of fibrinogen binding and the platelet-fibrin interaction contributing to clot retraction. Activation of platelet αIIbβ3 with an agonist results in αIIbβ3 being able to bind fibrinogen by the engagement of the γ-404-411 peptide in the αIIbβ3 binding pocket. EDTA can prevent the binding of fibrinogen and reverse the binding if added rapidly after the addition of fibrinogen. The binding of fibrinogen leads to conformational changes in fibrinogen that expose 1 or more ancillary binding sites. Platelet αIIbβ3 activation alone is sufficient to expose ancillary binding site(s), but there may be an additional contribution from fibrinogen engaging the αIIbβ3 binding pocket. The ancillary binding site(s) on fibrinogen and αIIbβ3 then interact, resulting in higher-affinity fibrinogen binding, which under certain experimental conditions may appear irreversible over the time course of the experiments. A similar mechanism may contribute to clot retraction, with fibrin formation initiating conformational changes in fibrinogen that mimic, at least in part, those induced by fibrinogen binding to αIIbβ3. Although data vary, the weight of evidence supports the need for activated platelets to participate in clot retraction. Paradoxically, EDTA treatment appears to initiate conformational changes in αIIbβ3 akin to those produced by activation and ligand binding as judged by the ability of cells expressing normal αIIbβ3 to bind to ‘D98’ in the presence of EDTA.