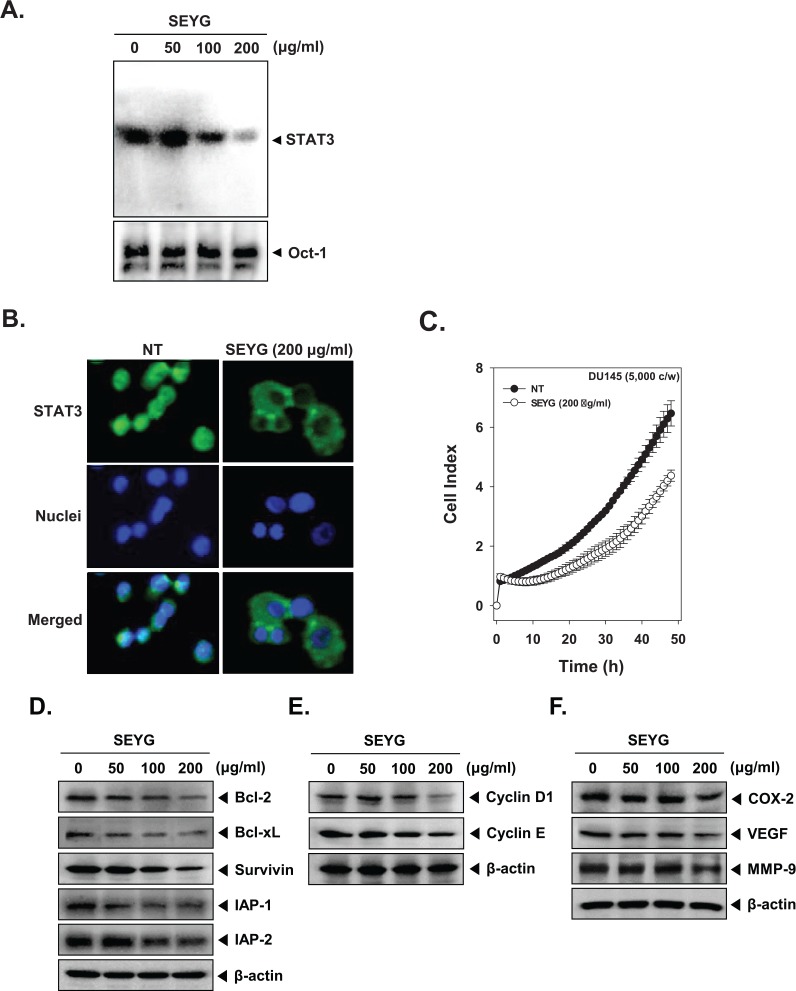
Figure 3.
SEYG inhibits binding of STAT3 to the DNA and expression of various gene products in human prostate cancer cells. (A) SEYG suppresses STAT3 binding activity. DU145 cells (1 × 106 cells/well) were treated with various indicated concentrations of SEYG for 6 hours, analyzed for nuclear STAT3 levels by EMSA. Oct-1 EMSA is shown as a loading control. (B) SEYG causes the inhibition of translocation of STAT3 to the nucleus. After 6 hours of SEYG treatment, the cells were fixed and permeabilized. STAT3 (green) was immunostained with rabbit anti-STAT3 followed by FITC-conjugated secondary antibodies and the nuclei (blue) were stained with DAPI. The third panels show the merged images of the first and second panels. The results shown are representative of 2 independent experiments. (C) Cell proliferation assay was performed using the Roche xCELLigence Real-Time Cell Analyzer (RTCA) DP instrument (Roche Diagnostics GmbH) as described in “Material and Methods.” After DU145 cells (5 × 103 cells/well) were seeded onto 96-well E-plates and continuously monitored using impedance technology. (D-F) DU145 cells (1 × 106 cells/well) were incubated with the indicated concentrations of SEYG for 24 hours. Whole-cell extracts were prepared, and 20 µg of the whole-cell lysate was resolved by SDS-PAGE, electrotransferred to nitrocellulose membrane, sliced from the membrane based on the molecular weight, and then probed with antibodies against bcl-2, bcl-xL, survivin, IAP1/2, cyclin D1, cyclin E, COX-2, VEGF, and MMP-9 as described in “Materials and Methods.” The same blots were stripped and reprobed with β-actin antibody to verify equal protein loading. The results shown here are representative of 3 independent experiments.