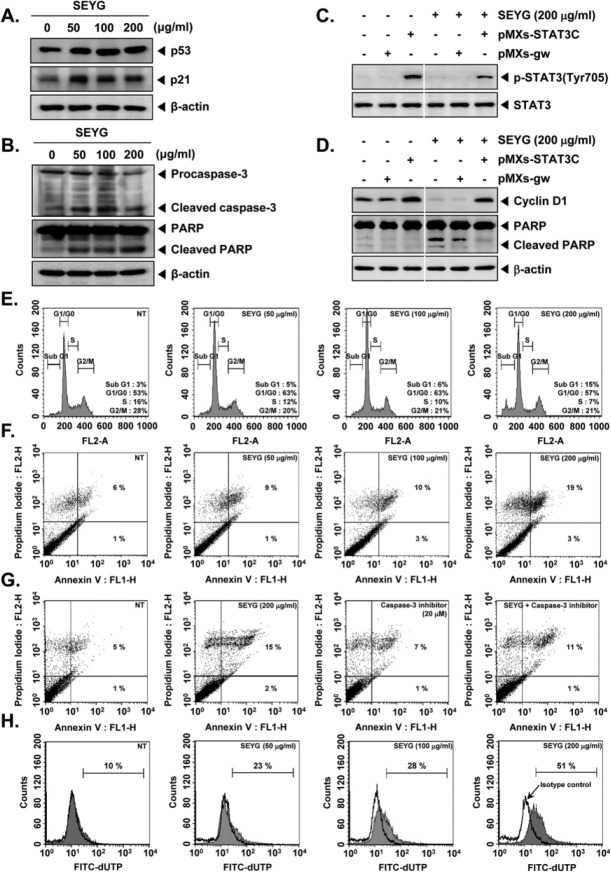
Figure 4.
SEYG induces apoptosis by PARP cleavage through activation of caspase-3. (A) DU145 cells (1 × 106 cells/well) were incubated with the indicated concentrations of SEYG for 24 hours. Whole-cell extracts were prepared, and 20 µg of the whole-cell lysate was resolved by SDS-PAGE, electrotransferred to nitrocellulose membrane, sliced from the membrane based on the molecular weight, and then probed with antibodies against p53 and p21 as described in “Materials and Methods.” The same blots were stripped and reprobed with β-actin antibody to verify equal protein loading. The results shown here are representative of 3 independent experiments. (B) After DU145 cells (1 × 106 cells/well) were seeded onto 6-well plates, they were treated with various indicated concentrations of SEYG for 24 hours. Thereafter, equal amounts of lysates were analyzed by Western blot analysis using antibodies against caspase-3 and PARP. The same blots were stripped and reprobed with β-actin antibody to verify equal protein loading. (C) MEF cells were transiently transfected with pMXs-STAT3C or pMXs-gw (control vector) plasmid. STAT3C protein was overexpressed in pMXs-STAT3C tranfected MEF cells compared to control. Transiently transfected cells were treated with indicated concentrations of SEYG for 6 hours. Then, equal amounts of lysate were analyzed by Western blot analysis using antibodies against phospho-STAT3 (Tyr705) and STAT3. (D) MEF cells were transiently transfected with pMXs-STAT3C or pMXs-gw (control vector) plasmid. STAT3C protein was overexpressed in pMXs-STAT3C tranfected MEF cells compared to control. Transiently transfected cells were treated with indicated concentrations of SEYG for 24 hours. Equal amounts of lysate were analyzed by Western blot analysis using antibody against cyclin D1 and PARP. The same blots were stripped and reprobed with β-actin antibody to verify equal protein loading. The results shown here are representative of 3 independent experiments. (E) After DU145 cells (1 × 106 cells/well) were seeded onto 6-well plates, they were incubated with the indicated concentrations of SEYG for 24 hours. Then, the cells were fixed and analyzed using flow cytometry. The results shown here are representative of 3 independent experiments. (F) Cells were treated with indicated concentrations of SEYG for 24 hours. Thereafter, they were incubated with anti-annexin V antibody conjugated with FITC plus PI and analyzed with a flow cytometer for apoptotic effects. The results shown here are representative of 3 independent experiments. (G) Cells were treated with indicated concentrations of SEYG and caspase-3 inhibitor for 24 hours. Thereafter, they were incubated with anti-annexin V antibody conjugated with FITC plus PI and analyzed with a flow cytometer for apoptotic effects. The results shown here are representative of 3 independent experiments. (H) Cells were treated with indicated concentrations of SEYG for 24 hours. Cells were fixed, stained with TUNEL assay reagent, and then analyzed with a flow cytometer.