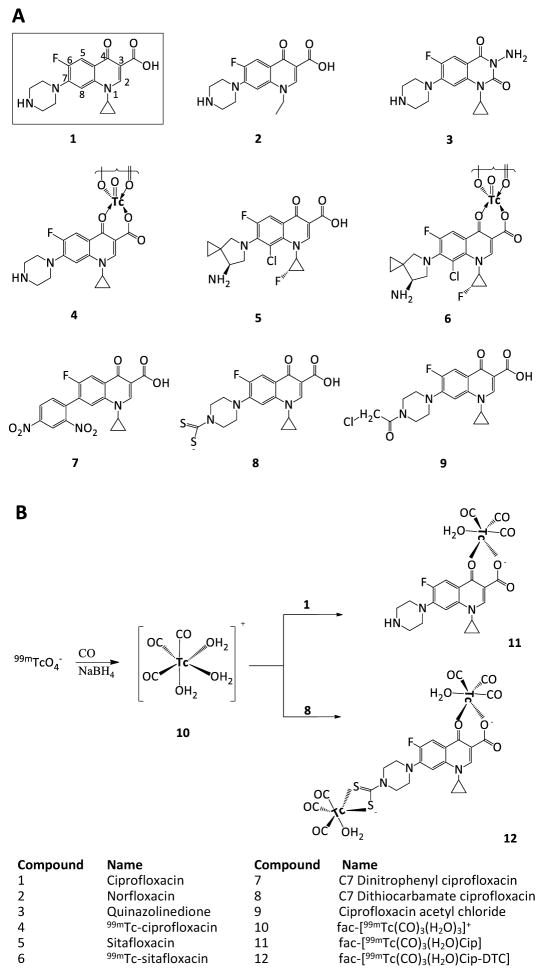
Figure 1.
Aspects of fluoroquinolone structure. Panel A. Structures of quinolone-class molecules. Quinolones have a characteristic dual-ring structure (numbering is as shown for ciprofloxacin). Ciprofloxacin differs from norfloxacin by replacement of the N1-ethyl with an N1-cyclopropyl group. The quinazolinedione shown is identical to ciprofloxacin except at the 2 and 3 positions. The absence of the C3 carboxyl prevents the dione from interacting strongly with amino acids in helix-4 of GyrA and ParC, which in turn makes diones insensitive to resistance substitutions in helix-4. 99mTc is shown interacting at the C3 carboxylate and C4 carbonyl substitutents of ciprofloxacin. Panel B. Labeling of ciprofloxacin (1) and dithiocarbamate ciprofloxacin (8) with fac-[99mTc(OH2)3(CO)3]+ (10). A carbonyl water complex of technetium (compound 10) is formed by reacting 99mTcO4 with carbon monoxide and sodium borohydride. Compound 10 then complexes with either ciprofloxacin (1) or compound 8, in both cases at the fluoroquinolone C3 and C4 positions.