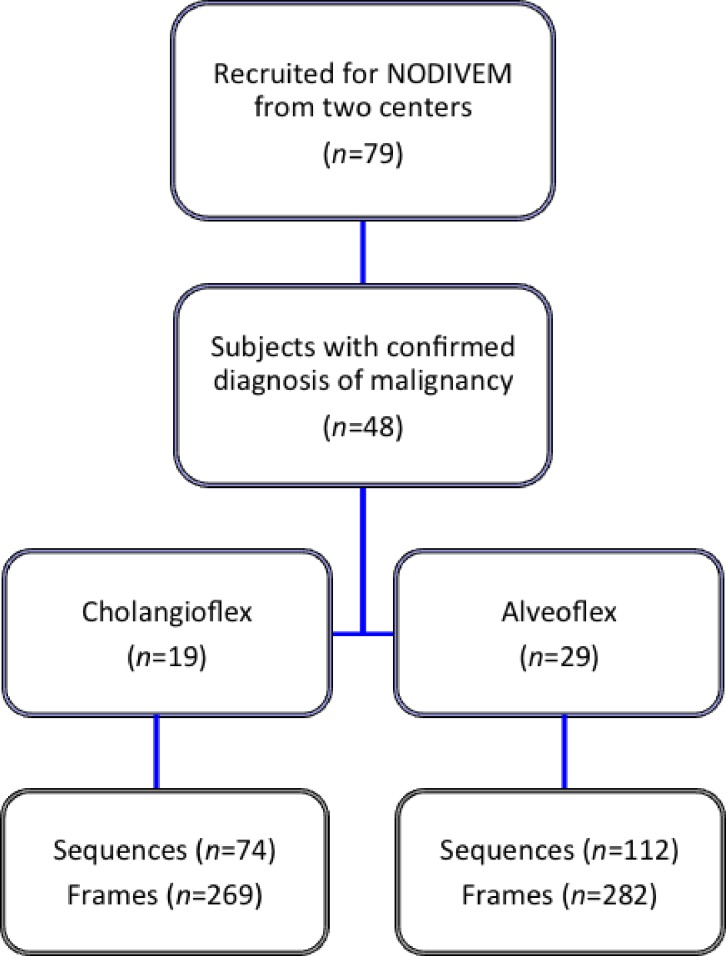
Fig 1. Flow chart of the study.
Subjects were recruited from either one of the two centers of the NODIVEM study. Inclusion criteria in the NODIVEM study included a final diagnosis of malignant SPN with histopathology confirmation and successful location of the SPN with r-EBUS. All of the patients signed a written informed consent before the pCLE procedure, which was performed with topical lidocaine but without conscious sedation.