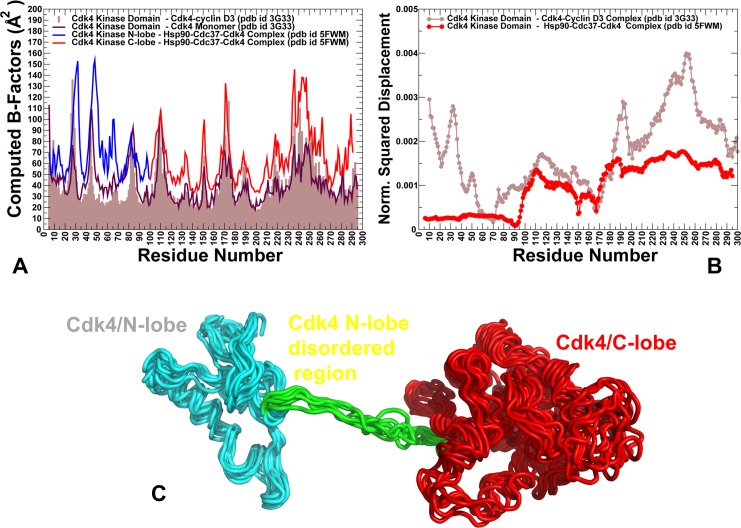
Fig 4. Conformational dynamics of Cdk4 in different functional states.
(A) Conformational dynamics profile of Cdk4 catalytic domain in the complex with cyclin D3 (pdb id 3G33) is shown in brown bars and serves a reference for comparison with mobility profiles in other functional states. Conformational dynamics profile of the unbound, monomeric Cdk4 form is shown in maroon lines. Cdk4 mobility in the Hsp90-Cdc37-Cdk4 complex is shown for N-lobe in blue lines) and C-lobe in red lines. (B) The normalized squared displacement of the Cdk4 domain residues averaged over first three PCA components are shown for the Cdk4-cyclin D3 complex (in brown lines) and for the Hsp90-Cdc37-Cdk4 complex (in red lines). (C) An overlay of representative Cdk4 conformations from the MD ensemble of the partially disordered Cdk4 client in the Hsp90-Cdc37-Cdk4 complex. The ordered portion of the N-lobe is shown in cyan ribbons, a disordered region of the N-lobe is in green ribbons, and the ordered C-lobe is in red ribbons. Notice a significant conformational mobility and inflated nature of the ordered regions, while disordered region is shielded by the Hsp90 interactions.