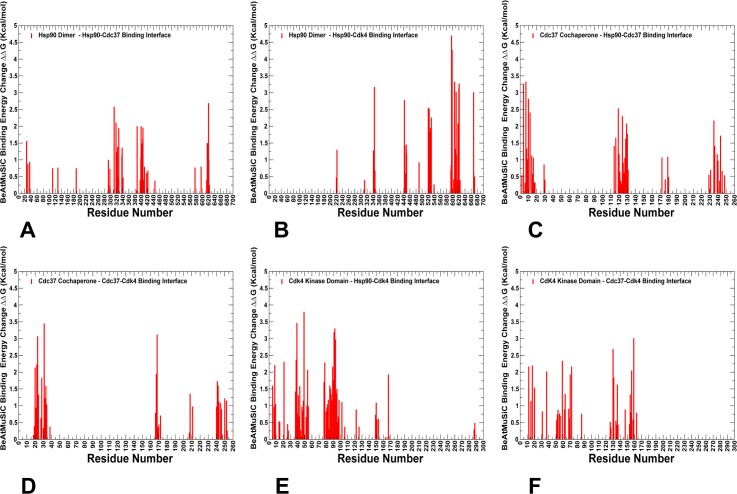
Fig 6. Alanine scanning and free energy analysis of the binding interfaces in the Hsp90-Cdc37-Cdk4 complex using BeAtMuSiC approach.
Binding free energy changes obtained through alanine scanning of the interfacial residues in the Hsp90-Cdc37-Cdk4 complex using BeAtMuSiC approach. Mutation-induced binding free energy changes ΔΔG of the Hsp90 residues involved in the Hsp90-Cdc37 interface (A) and Hsp90-Cdk4 interface (B). Binding free energy changes ΔΔG of the Cdc37 residues forming interfacial contacts with Hsp90 (C) and Cdk4 (D). Binding free energy changes ΔΔG of the Cdk4 residues contributing to the Cdk4-Hsp90 interface (E) and Cdk4-Cdc37 interface (F). If the free energy change between a mutant and the wild type (WT) proteins ΔΔG = ΔG (MT)-ΔG (WT) > 0, the mutation is considered to be destabilizing. The distributions are shown in red-colored bars and highlight only binding interface residues.