Figure 5.
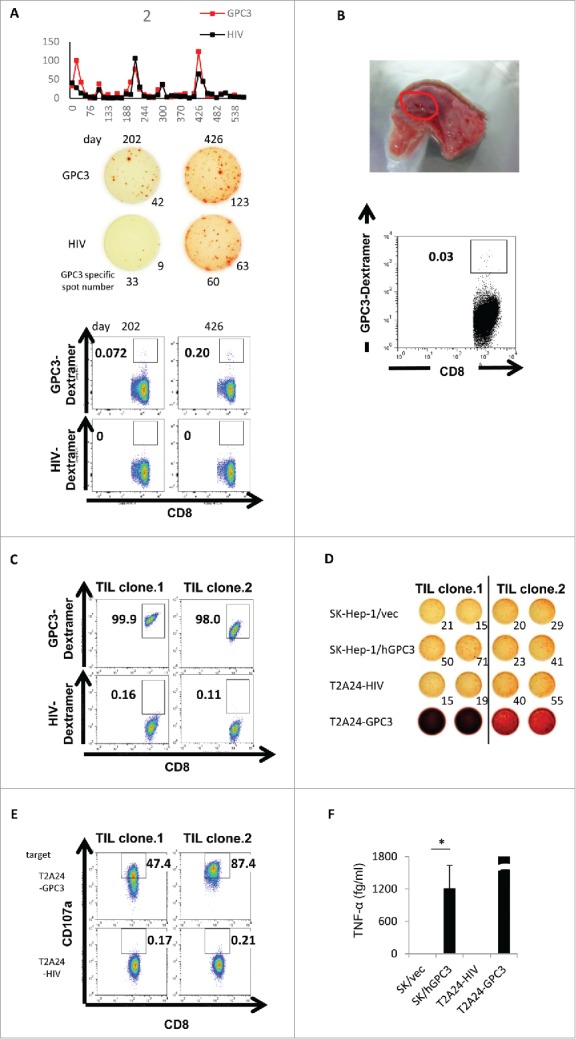
Immunological-response assessment in case 2. (A) The raw data of ex vivo IFN-γ ELISPOT assay using PBMCs of case 2. Dextramer assay using PBMCs of case 2 were performed after in vitro peptide stimulation. (B) Ex vivo GPC3-Dextramer staining after vaccination. GPC3-peptide-specific CTL frequency is indicated as the percentage of Dextramer-positive CTLs to CD8-positive cells in the tumor-resected specimen (red circle). (C) Dextramer analysis of the establishment of the GPC3-peptide-specific CTL clones in the tumor-biopsy specimen. (D) IFN-γ ELISPOT assay against SK-Hep-1/vec, SK-Hep-1/hGPC3, and peptide-pulsed T2A24. Effector / target (E / T) ratio = 0.2. (E) Externalized CD107a analysis of the establishment of the GPC3-peptide-specific CTL clones in the tumor-biopsy specimen. T2A24 pulsed with GPC3298–306 or HIV583–591 peptide were used as target cells. (F) TNF-α levels in the CTL clone (TIL clone.1) (1.0 × 105 cells/well) after a 24-h co-culture with the indicated target cells (5 × 104 cells/well). Data represent the mean ± standard deviation of triplicate cultures. *p < 0.05.
