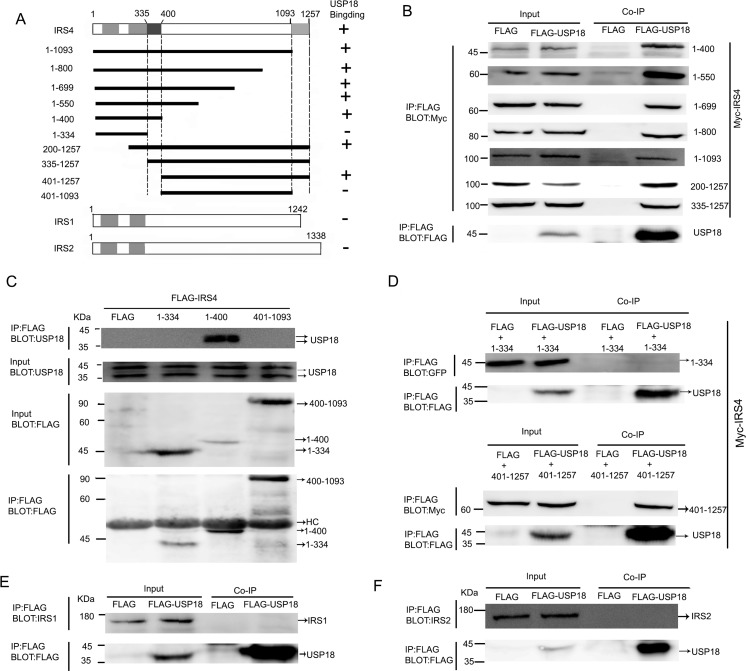
Figure 2. Mapping of USP18-binding regions of IRS4.
(A) Schematic depiction of human IRS4 and its deletion mutants used in this study. PH and PTB indicate the pleckstrin homology (PH) domain and phosphotyrosine-binding (PTB) domain, respectively. Summary of the binding domains of IRS4 with USP18 was listed on the right. A major USP18-binding region (amino acids 335–400 and 1094-1257) is indicated. (B–D) co-immunoprecipitation assays. (B) 293T cells co-expressing Flag-USP18 and Myc-IRS4 mutant's proteins were lysed and immunoprecipitated with an anti-Flag antibody. Immunoprecipitates (IP) and cell protein lysates (Input) were analyzed by immunoblotting with anti-Myc and anti-Flag antibodies. The figure shows only the bait protein of 1-400. (C) Co-IP assay of the interaction between three IRS4 mutants and endogenous USP18. Cell protein lysates were then immunoprecipitated with an anti-Flag antibody followed by immunoblotting with anti-USP18 and anti-Flag antibodies. HC, heavy chain. (D) 293T cells co-expressing Flag-USP18 and YFP-IRS4 mutant's proteins was lysed and immunoprecipitated with an anti-Flag antibody. Immunoprecipitates (IP) and cell protein lysates (Input) were analyzed by immunoblotting with anti-GFP and anti-Flag antibodies. (E, F) Co-IP assay of the interaction between USP18 and endogenous IRS1 and IRS2. Cell lysates were immunoprecipitated with an anti-Flag antibody followed by immunoblotting with anti-IRS1 or -IRS2(upper) and anti-Flag antibodies (lower).