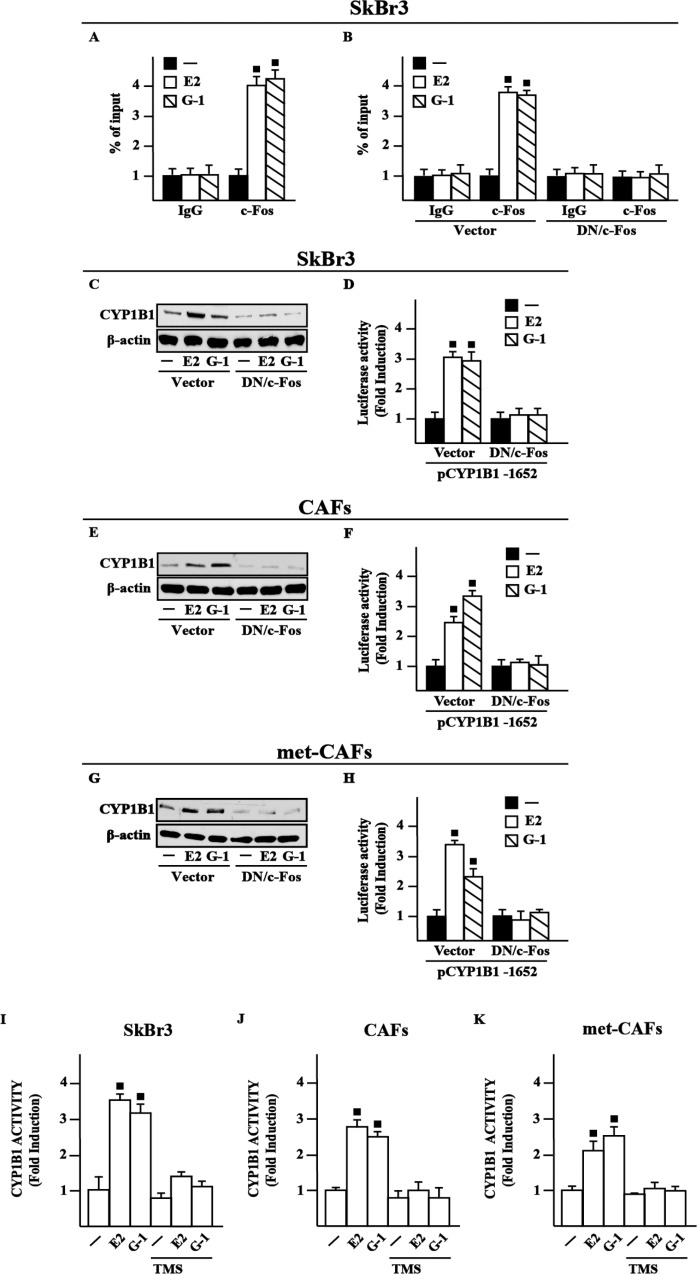
Figure 3. c-Fos is involved in the up-regulation of CYP1B1 by E2 and G-1 in SkBr3 cells, CAFs and met-CAFs.
(A) Recruitment of c-Fos induced by 10 nM E2 and 100 nM G-1 to the half-ERE site located within the CYP1B1 promoter sequence in SkBr3 cells. In control samples non-specific IgG was used instead of the primary antibody. (B) SkBr3 cells were transfected for 18 h with a vector or a construct encoding for a dominant negative form of c-Fos (DN/c-Fos), then treated for 3 h with vehicle (−), 10 nM E2 and 100 nM G-1 and thereafter submitted to the chromatin immunoprecipitation procedure using anti-c-Fos or nonspecific anti-IgG antibodies. The amplified sequences were evaluated by real-time PCR. CYP1B1 protein levels in SkBr3 cells (C), CAFs (E) and met-CAFs (G) transfected for 18 h with a vector or DN/c-Fos and then treated for 6 h with vehicle, 10 nM E2 and 100 nM G-1, as indicated. β-actin serves as a loading control. Results shown are representative of at least two independent experiments. SkBr3 cells (D), CAFs (F) and met- CAFs (H) were transfected for 18 h with a CYP1B1construct, a vector or DN/c-Fos and then treated for 18 h with vehicle, 10 nM E2 and 100 nM G-1. The luciferase activities were normalized to the internal transfection control and values of cells receiving vehicle were set as 1-fold induction upon which the activities induced by treatments were calculated. CYP1B1 activity evaluated by EROD assay in SkBr3 cells (I), CAFs (J) and met-CAFs (K) treated for 18 h with vehicle (−), 10 nM E2 and 100 nM G-1 alone or in combination with 5 μM CYP1B1 inhibitor TMS. Fluorescence values of cells receiving vehicle were set as 1-fold induction upon which values induced by treatments were calculated. Each column represents the mean ± SD for three independent experiments, each performed in triplicate. (■) indicates P < 0.05 for cells receiving treatments versus vehicle (−).