Figure 6.
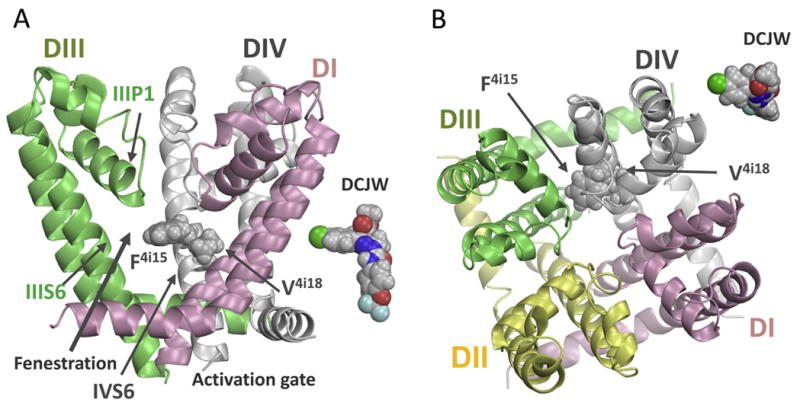
Side (A) and extracellular (B) views of the pore module in the NavAb-based homology model of the insect sodium channel BgNav1-1a. Domains DI, DII, DIII, and DIV are shown by pink, yellow, green, and gray ribbons, respectively. Domain DII is removed at the side view for clarity. Side chains of residues F4i15 and V4i18 are space-filled. For comparison, “side” and “top” views of a DCJW conformer are placed next to respective views of the channel. Mutations F4i15Y and V4i18I enlarge respective residues suggesting that DCJW binds tightly in the inner pore, forms close contacts with F4i15 and V4i18, and may expose its hydrophobic moiety to F4i15.
