Abstract
Plasma deoxy-sphingoid bases are elevated in type 2 diabetes patients and correlate with the stage of diabetic distal sensorimotor polyneuropathy; however, associations between deoxy-sphingolipids (DSL) and neuropathy in type 1 diabetes have not been examined. The primary aim of this exploratory pilot study was to assess the associations between multiple sphingolipid species including DSL and free amino acids and the presence of symptomatic neuropathy in a DCCT/EDIC type 1 diabetes subcohort. Using mass spectroscopy, plasma levels of DSL and free amino acids in DCCT/EDIC type 1 diabetes participants (n = 80), with and without symptoms of neuropathy, were investigated. Patient-determined neuropathy was based on 15-item self-administered questionnaire (Michigan Neuropathy Screening Instrument) developed to assess distal symmetrical peripheral neuropathy in diabetes. Patients who scored ≥4, or reported inability to sense their feet during walking or to distinguish hot from cold water while bathing were considered neuropathic. Plasma levels of ceramide, sphingomyelin, hexosyl- and lactosylceramide species, and amino acids were measured and analyzed relative to neuropathy status in the patient. Deoxy-C24-ceramide, C24- and C26-ceramide were higher in patients with neuropathy than those without neuropathy. Cysteine was higher in patients with neuropathy. No differences in other sphingolipids or amino acids were detected. The covariate-adjusted Odds Ratios of positive patient-reported neuropathy was associated with increased levels of deoxy-C24-, and deoxy-C24:1-ceramide; C22-, C24-, and C26-ceramide; and cysteine. Plasma deoxy-ceramide and ceramide species may have potential diagnostic and prognostic significance in diabetic neuropathy.
Keywords: Type 1 diabetes, Neuropathy, Deoxysphingolipid, Ceramide, Deoxy-ceramide, Cysteine
Introduction
Sphingolipids are heterogeneous lipids that are important constituents of cell membranes and plasma lipoproteins and which participate in a myriad of signaling functions (Zheng et al. 2006; Hannun and Obeid 2008). There is mounting evidence that sphingolipid metabolism is altered in diabetes, and specific sphingolipid classes may contribute to diabetic complications (Fox and Kester 2010; Galadari et al. 2013). Neuropathy is a detrimental chronic complication of diabetes mellitus. Clinically, diabetic neuropathy exhibits a pronounced similarity to the neuropathy of patients with hereditary sensory and autonomic neuropathy type 1 (HSAN1) and to the neuropathy due to chemotherapy. HSAN1 patients exhibit elevated plasma levels of a newly identified sphingolipid class, deoxysphingolipids (DSL) (Penno et al. 2010). In a recent study, plasma DSL levels have been shown to associate also with the incidence and severity of paclitaxel-induced peripheral neuropathy in breast cancer patients (Kramer et al. 2015). DSL were shown to have pronounced neurotoxic effects on neurite formation in cultured sensory neurons (Penno et al. 2010) and in phase I clinical trials where deoxy-sphinga-nine was tested for its chemotherapy potential; the trials were discontinued due to patients developing severe in some cases fatal neuropathy (Baird et al. 2009; Schöffski et al. 2011; Massard et al. 2012). Studies in an animal model of HSAN1 and in HSAN1 patients suggest that oral supplementation with L-serine reduces plasma DSL concentration (Garofalo et al. 2011) and raises the prospect of a treatment option to modulate plasma DSL levels. In addition, plasma levels of DSL are elevated in type 2 diabetes patients (Bertea et al. 2010; Othman et al. 2012; Wei et al. 2014) and in patients exhibiting symptoms of the metabolic syndrome (Othman et al. 2012). Levels of deoxy-sphingoid bases in type 1 diabetes patients were found not to be different from those in control subjects (Wei et al. 2014); however, no data are available on DSL levels in type 1 diabetes patients with neuropathy.
Genetic analysis of HSAN1 patients revealed at least four missense mutations in the genes coding for serine palmitolytransferase (SPT) subunits 1 and 2, the rate-limiting enzyme which regulates de novo sphingolipid biosynthesis (Bejaoui et al. 2001; Dawkins et al. 2001; Penno et al. 2010; Rotthier et al. 2010). The preferred substrates of SPT are the amino acid L-serine and palmitoyl-CoA. SPT catalyzes the condensation of L-serine and palmitoyl-CoA to form 3-keto-sphinganine, and ultimately sphinganine. Sphinganine is subsequently N-conjugated with a second fatty acid to form dihydroceramide. The majority of dihydroceramide is subsequently desaturated to form ceramide, the major building block for more complex sphingolipids (Bejaoui et al. 2001; Dawkins et al. 2001). SPT can utilize L-alanine or glycine as substrates, but with lesser preference. Using these alternate amino acid substrates, SPT activity generates an atypical category of sphingoid bases: the 1-deoxy-sphingoid bases. The conjugation of L-alanine forms the DSL deoxy-sphinganine; when glycine is used in the reaction, deoxy-methylsphinganine is formed (Rotthier et al. 2010). Because both of these sphingolipid metabolites do not contain the hydroxyl group normally located at C-1 in the sphinganine molecule formed when L-serine is used in the reaction catalyzed by SPT, these modified sphingolipids cannot be further metabolized to the more complex sphingolipids nor can they be degraded via the normal physiologic sphingolipid catabolic pathways to form 1-phosphate derivatives (Duan and Merrill 2015). Analysis of DSL in plasma revealed that DSL are present at low levels in plasma from normal, healthy individuals, primarily in very low density and low-density lipoproteins (Bertea et al. 2010). In HSAN1 patients, sphingolipid metabolism is altered due to gain-of-function mutations to the genes coding for SPT subunits 1 and 2. In these patients, L-alanine and L-glycine become the preferred amino acid substrates for the mutated SPT enzyme, which results in elevated levels of DSL (Bejaoui et al. 2001; Dawkins et al. 2001; Rotthier et al. 2010). Moreover, it was shown that the anticancer drug paclitaxel can upregulate SPT protein levels and activity, which also results in elevated levels of DSL (Kramer et al. 2015).
The Diabetes Control and Complications Trial (DCCT) was a longitudinal intervention study of 1441 subjects with type 1 diabetes (DCCT Research Group 1993) aged 13–39 years with 1- to 15-year diabetes duration and with mild or no diabetic complications at study entry (1983–1989). Some patients were free from clinically evident retinopathy (primary cohort, Early Treatment Diabetic Retinopathy Study score, ETDRS = 1, AER ≤ 30 mg/24 h, diabetes duration 1–5 years), some had mild-to-moderate nonproliferative diabetic retinopathy (secondary cohort, ETDRS 2–9, AER ≤ 300 mg/24 h, diabetes duration 1–15 years). The participants were randomized into two treatment groups that received either intensive or conventional insulin therapy and were followed for an average of 6.5 years. At baseline DCCT examination, each participant received a complete physical examination which included a medical history, an electrocardiogram, and routine laboratory analyses to determine serum creatinine, AER, lipid profile, and HbA1c levels (DCCT Research Group 1993). The DCCT was stopped ahead of schedule in 1993 because of the observed major beneficial effect of intensive therapy on retinal, renal, and neurologic complications. The DCCT was continued as an observational study (1994–present), the Epidemiology of Diabetes Interventions (EDIC) study, and 96 % of the subjects originally enrolled in the DCCT chose to participate (EDIC Research Group 1999). The goal of the EDIC study was to assess long-term effects of prior separation of glycemic levels on micro- and macrovascular outcomes in type 1 diabetes (EDIC Research Group 1999).
Herein, we investigated the association of plasma levels of sphingolipids including DSL with diabetic neuropathy in type 1 diabetes patients in a DCCT/EDIC subcohort and conducted companion analyses to determine differences in plasma free amino acid levels with neuropathy using a cross-sectional study design.
Materials and Methods
Study Subjects
We determined the plasma concentrations of free amino acids and sphingolipids including DSL using banked samples obtained between year 3 and year 7 of the EDIC study (1997–2001) from 19 type 1 diabetic patients enrolled in the DCCT/EDIC cohort who exhibited symptoms of neuropathy using the criteria detailed below and compared their plasma amino acids and sphingolipid levels to those in 61 control type 1 diabetes patients who were classified to be free from diabetic neuropathy symptoms at the time of sampling. If sufficient volume of a plasma sample collected at EDIC year 4 was not available to conduct all the analyses required, a sample collected from the same patient during the subsequent year was used for analysis (Table 1). Samples were collected from the population of the DCCT/EDIC cohort that had (1) available sample for sphingolipid measurement, (2) MNSI data collection within 12 months of the date of the sample collection, and (3) a normal lipid profile (LDL ≤ 130, HDL > 40, and triglycerides ≤ 150) at the time of sample collection. Samples were not restricted based on any other comorbid conditions.
Table 1. Demographics and clinical characteristics by patient-reported neuropathy symptomology during EDIC.
| Demographics and clinical characteristics | Patient-reported neuropathy symptomologya | p valueb | |
|---|---|---|---|
|
|
|||
| Symptomatic (N = 19) | Not symptomatic (N = 61) | ||
| DCCT baseline demographics and clinical characteristics | |||
| Male% (n) | 52.6 (10) | 42.6 (26) | 0.444 |
| Intensive DCCT treatment assignment% (n) | 31.6 (6) | 36.1 (22) | 0.720 |
| Primary disease cohort% (n) | 47.4 (9) | 37.7 (23) | 0.453 |
| Age (years) | 28.6 ± 7.7 | 28.1 ± 6.9 | 0.689 |
| Duration of type 1 diabetes (years) | 5.5 ± 3.6 | 6.1 ± 3.9 | 0.624 |
| HbA1c% | 9.5 ± 1.6 | 8.5 ± 1.4 | 0.030 |
| Albumin excretion rate (AER) | 20.8 ± 13.8 | 12.7 ± 8.6 | 0.026 |
| LDL cholesterol (mg/dl) | 108.6 ± 27.2 | 99.8 ± 20.5 | 0.371 |
| HDL cholesterol (mg/dl) | 51.4 ± 8.7 | 55.4 ± 11.4 | 0.195 |
| Triglycerides (mg/dl) | 76.3 ± 36.9 | 65.6 ± 14.3 | 0.421 |
| Serum creatinine (mg/dl) | 0.8 ± 0.2 | 0.8 ± 0.2 | 0.210 |
| EDIC year 5 demographics and clinical characteristics | |||
| Age (years) | 41.8 ± 8.2 | 41.6 ± 6.5 | 0.808 |
| Duration of type 1 diabetes (years) | 18.3 ± 4.4 | 19.3 ± 4.8 | 0.518 |
| HbA1c% | 8.7 ± 1.7 | 7.9 ± 1.2 | 0.067 |
| Mean EDIC HbA1c% | 8.9 ± 1.5 | 8.0 ± 1.0 | 0.034 |
| Albumin excretion rate (AER) | 117.6 ± 242.4 | 21.5 ± 42.2 | 0.067 |
| ETDRS score | 5.5 ± 3.0 | 4.0 ± 2.2 | 0.031 |
| Any clinically sig. macular edema% (n) | 15.8 (3) | 6.8 (4) | 0.352 |
| Any sig. nonproliferative diabetic retinopathy% (n) | 5.3 (1) | 6.8 (4) | 0.991 |
| LDL cholesterol (mg/dl) | 104.6 ± 11.3 | 100.9 ± 19.2 | 0.299 |
| HDL cholesterol (mg/dl) | 58.8 ± 14.3 | 63.5 ± 14.2 | 0.109 |
| Triglycerides (mg/dl) | 68.3 ± 27.5 | 67.6 ± 30.2 | 0.539 |
| Any ACE inhibitor use% (n) | 42.1 (8) | 41.0 (25) | 0.931 |
| Any statin drug use% (n) | 15.8 (3) | 18.0 (11) | 0.822 |
Italicized bold numbers indicate p < 0.05
The MNSI was not administered during DCCT; its use started with EDIC
p value as determined by nonparametric Wilcoxon rank-sum test or Pearson Chi-square test statistics (Fisher's exact test where necessary)
Neuropathy Classification
Neuropathy status in each DCCT/EDIC study subject is evaluated annually (Feldman et al. 1994; EDIC Research Group 1999) and for the purposes of this pilot project, neuropathy was determined based on the results of the Michigan Neuropathy Screening Instrument (MNSI) which is administered annually to each study subject (Dyck et al. 1986). The MNSI includes two separate assessments—the first is a 15-item, self-administered questionnaire, while the second is a physical examination of the lower extremities which is administered by diabetes care personnel at each study site (Feldman et al. 1994; EDIC Research Group 1999). In this study, the neuropathy “positive” outcome status was adapted from Herman et al. (2012). Patients were considered positive for neuropathy symptoms if their score on the MNSI questionnaire was greater than or equal to 4.0, or if they responded “NO” to either question 7 (“When you get into the tub or shower, are you able to tell the hot water from the cold water?”) or question 13 (“Are you able to sense your feet when you walk?”) on the MNSI questionnaire. The results of the clinical examination were not considered during classification of neuropathy status for this study. Thus, plasma sphingolipid levels were determined in samples collected from patients who exhibited concurrent neuropathy symptoms based on self-assessment.
Plasma Sphingolipid and Amino Acid Analysis
Fasting blood samples were collected using EDTA as anticoagulant, and plasma was immediately separated and subsequently stored at −70 °C until the sample was thawed to enable the analysis of sphingolipid content. Only a primary, previously unthawed plasma aliquot was used to assay of plasma sphingolipid concentrations.
Analysis of plasma levels of DSL (deoxy-sphingosine, deoxy-dihydro-sphingosine, and deoxy-ceramides) and L-serine-derived sphingolipids (sphingoid bases, sphingoid base 1-phosphates, ceramides, sphingomyelins, hexosyl-, and lactosylceramides) were conducted in the Lipidomics Core Facility at the Medical University of South Carolina as previously described (Bielawski et al. 2009; Hammad et al. 2010; Kramer et al. 2015). Briefly, for analyses of L-serine-derived sphingolipids, 100 μl of plasma from each patient was fortified with internal standards as described previously (Hammad et al. 2010). Due to interferences from the internal standards used for quantification of 18Csphingosine and ceramide co-eluting with deoxy-sphingoid bases and their ceramides, a separate 100 μl of plasma from each patient was used for the determination of deoxysphingoid bases and deoxy-ceramide species. The samples for DSL extraction were fortified with internal standards as described previously (Kramer et al. 2015).
Sphingolipids were extracted with 2 ml extraction solution consisting of ethyl acetate/isopropanol (85/15) solvent system using vortexing and centrifugation for 5 min at 3000 rpm (2500g) on a Beckman Allegra 6R Centrifuge (Beckman Coulter, Brea, CA) (Hammad et al. 2010). The upper organic phase was then transferred to an 8-ml glass tube. The remaining diluted plasma was then acidified with 100 μl formic acid (98 %), and an additional 2 ml of extraction solution was added to further facilitate completion of extraction. The samples were then vortexed and centrifuged for 5 min at 3000 rpm (2500g). The upper organic phase was then transferred and added to the glass tube containing the initial extract and mixed by vortexing. The extract was evaporated to dryness with an N-Evap™ 112 nitrogen evaporator (Organomation Associates, Berlin, MA) (Hammad et al. 2010). After evaporation and reconstitution in 150 μl methanol, the DSL and L-serine-derived sphingolipids were separated using high-performance liquid chromatography-tandem mass spectrometry (HPLCESI-MS/MS) as described previously (Bielawski et al. 2009; Hammad et al. 2010; Kramer et al. 2015). Briefly, samples were injected into a Thermo TSQ Quantum LC/MS system and chromatographic separations were obtained under a gradient elution, using mobile phase A consisting of 2 mM ammonium formate in 0.2 % formic acid in water, and mobile phase B consisted of 1 mM ammonium formate in 0.2 % formic acid in methanol, on a Peeke Scientific (Redwood City, CA) Spectra C8SR 150 × 3.0 mm 3-lm-particle size column. Amino acids were analyzed on a Kinetex C18, 100 × 3.0; 2.6 μm column. Quantitative analyses were based on calibration curves generated by spiking an artificial matrix with known amounts of the synthetic standards for target analytes of interest, and an equal amount of the appropriate internal standards. The target analyte/internal standard peak areas ratios were plotted against the analyte concentration. The target analyte/internal standard peak area ratios from the samples were similarly normalized to their respective internal standards and compared to the calibration curves, using a linear regression model (Bielawski et al. 2009; Hammad et al. 2010). Peaks corresponding to the target analytes and internal standards were collected and processed using the Thermo Fisher software package Xcalibur 4.0. The resulting data were then normalized to the volume of sample analyzed.
The sphingolipid calibration standards were obtained from the MUSC Lipidomics Share Resource facility, and/ or from commercially available sources, including Avanti Polar Lipids Inc. and Matreya LLC. Sphingolipids, for which no synthetic standards are available, were quantified using the calibration curve of the closest eluting counterpart as described previously (Hammad et al. 2010; Kramer et al. 2015). For amino acids, eight-point calibration were constructed using “Amino Acid Standard Mix H” from Thermo Scientific (prod #20088).
Statistical Analysis
The primary aim of this exploratory pilot study was to assess the associations between multiple sphingolipid species and free amino acids and the presence of symptomatic neuropathy in a DCCT/EDIC type 1 diabetes subcohort. The concentrations of amino acids, DSL and L-serine-derived sphingolipid species were measured in plasma and were associated with concurrently collected patient-reported neuropathy status. As this was a pilot study, the sample size was not chosen to optimize power for statistical significance or multiple testing. The objective was to provide evidence of a signal so that a larger, more definitive study can be undertaken. Standard descriptive statistics were used to summarize the general demographic and clinical data at DCCT baseline and again at the time point concurrent with the sphingolipid and amino acid measures. The Kruskal–Wallis test was used to evaluate continuous demographic and clinical measures across neuropathy outcomes; the Pearson's Chi-square test (or Fisher's exact test) was used to assess the association of categorical variables. Initial, unadjusted sphingolipid and amino acid concentrations are noted as means and associated standard errors and are compared across neuropathy outcomes using Wilcoxon rank-sum test statistics.
The primary statistical model was developed to assess the association of the neuropathy classification as the dependent variable and the amino acid or sphingolipid species as the primary independent variable. Logistic regression models were used to quantify the association of increased marker levels on the subsequent patient-reported neuropathy. The primary parameter of interest in the logistic regression models was the change in the log-odds (with 95 % Wald CI) for the diagnosis of neuropathy as compared to those that remained without neuropathy; initial design models were adjusted for mean study HbA1c% as well as the baseline disease cohort. Additionally, baseline characteristics were tested for univariate prognostic association with neuropathy status using simple logistic regression models. Covariates significantly associated with the neuropathy outcomes were included in covariate-adjusted models. All models were additionally adjusted for concurrent lipid levels (LDL, HDL, triglycerides). In order to yield stable estimates with sparse data, all marker levels were normalized (log-transformed) and standardized (z score) such that the analysis results represented the association between a one standard deviation change in each biomarker and the odds to self-report neuropathy symptoms. All statistical analyses were performed using the SAS System version 9.3. Significance for all planned pilot study comparisons was set at a 2-sided p value of 0.05, and no correction for multiple testing was applied to reported p values.
Results
The concentrations of multiple sphingolipid species were measured in plasma samples collected between 1997 and 2001 from 80 participants enrolled in the EDIC study. Characteristics of study participants with and without patient-reported neuropathy are summarized in Table 1. Baseline measures of age and cholesterol (LDL, HDL, and triglycerides) were similar between the two groups; however, those with neuropathy symptomology had increased baseline levels of AER (20.8 ± 13.8 vs. 12.7 ± 8.6, p = 0.026) and HbA1c% (9.5 ± 1.6 vs. 8.5 ± 1.4, p = 0.030) as compared to those without. The time taken for the follow-up measurement was approximately 13.1 ± 0.3 years following the start of DCCT and did not differ between the group of patients with neuropathy compared to those without neuropathy (13.0 ± 0.5 vs. 13.1 ± 0.3, p = 0.927). At EDIC year 5, lipid levels in plasma from the two groups remained similar (p > 0.10), and those with reported neuropathy symptomology had moderately higher mean HbA1c% during EDIC follow-up (8.9 ± 1.5 vs. 8.0 ± 1.0, p = 0.034). There were no group differences or predictive associations with the use of ACE inhibitors or statin drugs (p = 0.931 and p = 0.822, respectively). While there was no difference in the prevalence of any significant non-proliferative diabetic retinopathy (p = 0.991) or clinically significant macular edema (p = 0.352), EDTRS scores were slightly higher in those with self-reported neuropathy (5.5 ± 3.0 vs. 4.0 ± 2.2, p = 0.031). Although concurrent ETDRS scores, mean HbA1c% and concurrent AER were significantly associated with neuropathy status, it was determined that all were comorbid outcomes significantly related to the participants randomized DCCT treatment assignment (as well as collinear with each other). Mean EDIC HbA1c% was the strongest univariate predictor of reported neuropathy and was retained in the analysis model. Concurrent levels of LDL and triglycerides were positively associated with some measured ceramides and deoxy-ceramides (all ρ < 0.50, p < 0.01), but were not associated with the measured amino acids, hexosylceramides, lactosylceramides or sphingomyelins. Models were assessed in a stepwise manner to determine any effects of collinearity on model fit with the addition of the lipid measures. There were no instances where significant relationships between sphingolipid and DSL species with neuropathy outcomes were masked by the inclusion of the lipid measures in the model (significant collinearity). The sampled cohort was additionally compared to the remaining DCCT/EDIC cohort on baseline levels of HbA1c, AER, retinopathy severity level, serum creatinine, duration of diabetes, lipid levels, age, percentage of subjects in primary cohort, sex and randomized treatment assignment. Participants in the study cohort were more likely to have lower LDL and triglycerides (LDL: 102 ± 22 vs. 110 ± 29, p = 0.010; and triglycerides: 68 ± 24 vs. 82 ± 48, p = 0.008) and increased levels of HDL (54 ± 11 vs. 50 ± 12, p < 0.001) at baseline. Additionally, those selected for the study were less likely to be in the intensive therapy treatment group (35 vs. 50 %, p = 0.008) and more likely to be in the secondary disease cohort (40 vs. 51 %, p = 0.056).
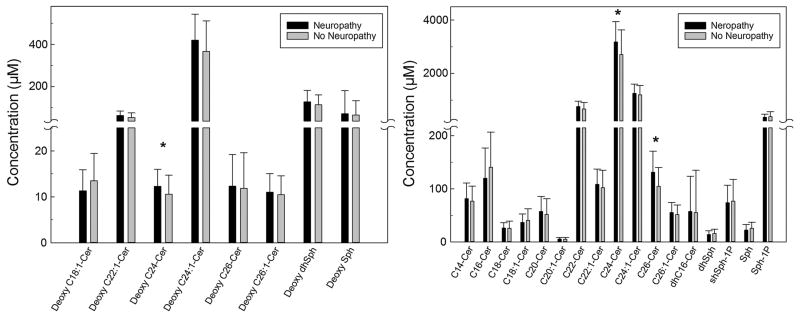
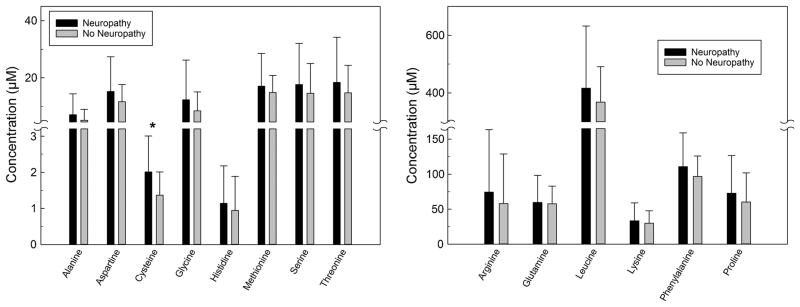
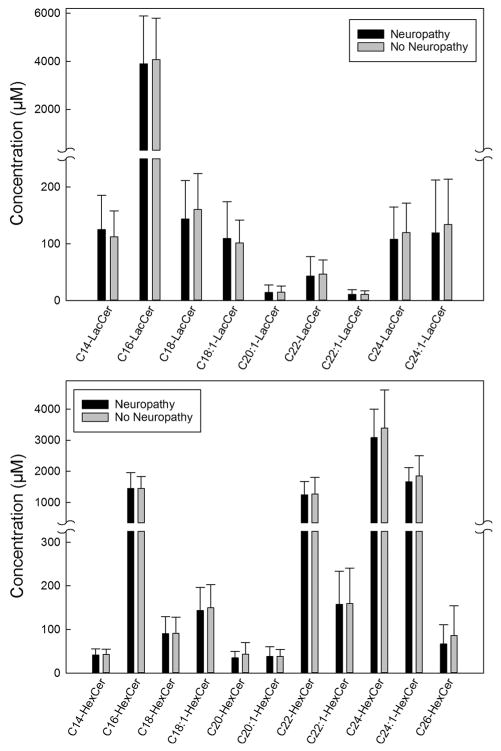
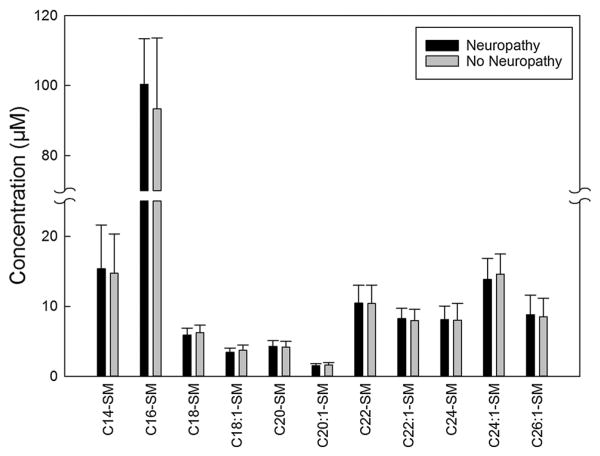
The unadjusted mean concentrations of sphingolipids and amino acids measured in the plasma samples obtained from type 1 diabetes patients with and without neuropathy are summarized in Figs. 1, 2, 34. Plasma levels of deoxyC24-ceramide (Fig. 1, left panel), C24- and C26-ceramide (Fig. 1, right panel) were significantly higher in patients with neuropathy compared to levels in those without neuropathy (12.3 ± 3.7 vs. 10.6 ± 4.1, p = 0.049; 3184.6 ± 762.7 vs. 2709.5 ± 921.8, p = 0.039; and 131.1 ± 39.7 vs. 104.6 ± 35.6 nM, p = 0.014). Plasma levels of individual amino acids were measured, and cysteine was higher in patients with neuropathy (2.0 ± 1.0 vs. 1.4 ± 0.6 μM, p = 0.007) (Fig. 2, left panel). No differences in other amino acids were detected (Fig. 2). There were no significant differences in the plasma concentrations of lactosylcermide species (Fig. 3, upper panel), hexosylceramide species (Fig. 3, lower panel), or sphingomyelin species (Fig. 4) between patients with self-reported neuropathy and those without.
Fig. 1.
Concentrations of deoxy-ceramide and ceramide species measured in 100 μl plasma samples obtained from type 1 diabetes patients with (n = 19) and without (n = 61) self-reported neuropathy using mass spectroscopy. Left panel deoxy-ceramide species; right panel ceramide species. Data presented as mean + standard deviation; *p < 0.05
Fig. 2.
Concentrations of amino acids species measured in 100 μl plasma samples obtained from type 1 diabetes patients with (n = 19) and without (n = 61) self-reported neuropathy using mass spectroscopy. Left panel amino acids with concentrations <40 μM; right panel amino acids with higher μM concentrations. Data presented as mean + standard deviation; *p = 0.007
Fig. 3.
Concentrations of lactosylcermide species and hexosylceramide species measured in 100 μl plasma samples obtained from type 1 diabetes patients with (n = 19) and without (n = 61) self-reported neuropathy using mass spectroscopy. Upper panel lactosylcermide species; lower panel hexosylceramide species. Data presented as mean + standard deviation
Fig. 4.
Concentrations of sphingomyelin species measured in 100 μl plasma samples obtained from type 1 diabetes patients with (n = 19) and without (n = 61) self-reported neuropathy using mass spectroscopy. Data presented as mean + standard deviation
The adjusted Odds Ratios of having positive patient-reported neuropathy symptomology with an increase in the plasma level of individual amino acid and sphingolipid species are shown in Tables 2 and 3. In covariates-adjusted models (i.e., baseline disease cohort, mean EDIC HbA1c%, and concurrent LDL, HDL, and triglycerides), increases in plasma levels of total ceramides and total deoxy-ceramides were associated with increased odds to report concurrent neuropathy symptomology [OR 2.70 (1.11–6.59), p = 0.029 and OR 2.92 (1.20–7.13), p = 0.018, respectively]. These differences in total levels were primarily driven by several individual species. Increased levels of deoxy-C24- and deoxy-C24:1-ceramide were associated with greater than twofold increases in the odds to report concurrent neuropathy symptomology [OR 2.9 (1.3–6.9), p = 0.014 and 3.1 (1.2–7.9), p = 0.016, respectively]. Similarly, increased levels of C22-, C24- and C26-ceramide were also associated with a significant increase in the odds to report concurrent neuropathy symptomology [OR 2.4 (1.1–5.4), p = 0.028; 2.8 (1.2–6.6), p = 0.019; and 3.6 (1.3–9.6), p = 0.011, respectively] and the amino acid cysteine [OR 2.3 (1.2–4.4), p = 0.010]. There were no significant effects of increased levels of any of the lactosylceramide species, hexosylceramide species or sphingomyelin species on the odds of developing self-reported neuropathy (all p > 0.05). The Odds Ratio of negative patient-reported neuropathy symptomology was associated with increased sphingosine (p < 0.05). Although not specifically powered to assess interactions, in this study cohort, there were no significant modifying effects of DCCT treatment assignment or the baseline disease cohort on the relationships between measured markers and reported neuropathy.
Table 2. Odds of self-reported neuropathy diagnosis for patients with increased plasma levels of sphingolipid species.
| Deoxy-ceramides | Deoxy-sphingosines | Ceramides | Sphingosines | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Species | Odds Ratio | Molecule | Odds Ratio | Species | Odds Ratio | Molecule | Odds Ratio |
| Deoxy C18:1 | 0.57 (0.30–1.08) | Deoxy dhSph | 1.31 (0.74-2.30) | C14 | 1.57 (0.76–3.23) | dhSph | 0.54 (0.28–1.01) |
| Deoxy C22:1 | 1.92 (0.94–3.93) | Deoxy Sph | 1.00 (0.55–1.85) | C16 | 0.78 (0.41–1.48) | dhSph-1P | 1.22 (0.69–2.17) |
| Deoxy C24 | 2.93 (1.29–6.93) | C18 | 0.96 (0.52–1.77) | Sph | 0.48 (0.22–1.03) | ||
| Deoxy C24:1 | 3.14 (1.49–7.94) | C18:1 | 0.80 (0.42–1.54) | Sph-1P | 1.10 (0.62–1.96) | ||
| Deoxy C26 | 1.36 (0.69–2.67) | C20 | 1.53 (0.77–3.04) | ||||
| Deoxy C26:1 | 1.69 (0.82–3.50) | C20:1 | 1.11 (0.59–2.08) | ||||
| Total deoxy-cer | 2.93 (1.20–7.13) | C22 | 2.44 (1.10–5.38) | ||||
| C22:1 | 1.20 (0.62–2.34) | ||||||
| C24 | 2.79 (1.18–6.60) | ||||||
| C24:1 | 1.67 (0.75–3.74) | ||||||
| C26 | 3.59 (1.34–9.62) | ||||||
| C26:1 | 1.38 (0.72–2.62) | ||||||
| dhC16 | 1.94 (0.97–3.87) | ||||||
| Total Cer | 2.70 (1.23–6.59) | ||||||
N = 19 patient-reported neuropathy during EDIC
Results from logistic regression models
Data are presented as the Odds Ratio and associated 95 % CI
Odds Ratio >1 detrimental effect, <1 protective effect
Analyses adjusted for mean EDIC HbA1c%, baseline disease cohort, concurrent LDL, HDL and triglycerides
Italicized bold numbers indicate p < 0.05
Table 3. Odds of self-reported neuropathy diagnosis for patients with increased plasma levels of sphingolipid species and amino acids.
| Hexosylceramides | Lactosylceramides | Sphingomyelins | Amino acids | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Species | Odds Ratio | Species | Odds Ratio | Species | Odds Ratio | Molecule | Odds Ratio |
| C14 | 1.01 (0.59–1.74) | C14 | 1.63 (0.88–3.02) | C14 | 1.17 (0.63–2.20) | Alanine | 1.08 (0.63–1.87) |
| C16 | 1.17 (0.63–2.17) | C16 | 0.91 (0.52–1.60) | C16 | 1.59 (0.81–3.12) | Arginine | 1.15 (0.65–2.03) |
| C18 | 1.03 (0.59–1.82) | C18 | 0.77 (0.42–1.41) | C18 | 0.71 (0.39–1.30) | Aspartic acid | 1.26 (0.74–2.15) |
| C18:1 | 0.79 (0.44–1.40) | C18:1 | 1.13 (0.61–2.11) | C18:1 | 0.68 (0.38–1.21) | Cysteine | 2.32 (1.23–4.37) |
| C20 | 0.76 (0.43–1.37) | C20:1 | 0.77 (0.44–1.33) | C20 | 1.19 (0.63–2.22) | Glutamine | 0.74 (0.44–1.22) |
| C20:1 | 0.95 (0.55–1.66) | C22 | 0.78 (0.42–1.45) | C20:1 | 0.69 (0.36–1.33) | Glycine | 1.00 (0.57–1.74) |
| C22 | 1.07 (0.58–1.98) | C22:1 | 0.85 (0.44–1.62) | C22 | 0.97 (0.52–1.80) | Histidine | 1.07 (0.61–1.86) |
| C22:1 | 1.09 (0.59–2.01) | C24 | 0.75 (0.42–1.34) | C22:1 | 1.10 (0.56–2.14) | Leucine | 1.23 (0.72–2.12) |
| C24 | 0.86 (0.48–1.55) | C24:1 | 0.72 (0.40–1.31) | C24 | 1.18 (0.62–2.25) | Lysine | 0.92 (0.54–1.59) |
| C24:1 | 0.79 (0.44–1.42) | Total lacto | 0.90 (0.51–1.59) | C24:1 | 0.68 (0.36–1.26) | Methionine | 1.08 (0.62–1.89) |
| C26 | 0.60 (0.32–1.12) | C26:1 | 0.93 (0.51–1.70) | Phenylalanine | 1.43 (0.83–2.47) | ||
| Total hexo | 0.89 (0.49–1.61) | Total SM | 1.42 (0.69–2.92) | Proline | 1.06 (0.61–1.84) | ||
| Serine | 1.08 (0.63–1.83) | ||||||
| Threonine | 1.10 (0.64–1.88) | ||||||
| Tyrosine | 1.23 (0.71–2.16) | ||||||
| Valine | 1.21 (0.70–2.09) | ||||||
| Total AA | 1.14 (0.66–1.98) | ||||||
N = 19 patient-reported neuropathy during EDIC
Results from logistic regression models
Data are presented as the odds Ratio and associated 95 % CI
Odds Ratio >1 detrimental effect, <1 protective effect
Analyses adjusted for mean EDIC HbA1c%, baseline disease cohort, concurrent LDL, HDL and triglycerides
Italicized bold numbers indicate p < 0.05
Discussion
There is limited information regarding the distribution of sphingolipids in plasma from type 1 diabetes patients with complications. We investigated the plasma concentrations of DSL, L-serine-derived sphingolipids and free amino acids in a subgroup of DCCT/EDIC type 1 diabetes patients, with and without symptoms of neuropathy. Neuropathy was defined by self-report of symptoms using a 15-item self-administered MNSI questionnaire (Herman et al. 2012). We determined that plasma levels of deoxyC24-ceramide, C24- and C26-ceramide, and cysteine were higher in patients with neuropathy than those without neuropathy. No differences in the concentrations of the other measured sphingolipids or in plasma amino acid concentrations were detected. The Odds Ratio of a positive patient-reported neuropathy diagnosis was associated with increased levels of deoxy-C22:1-, deoxy-C24- and deoxyC24:1-ceramide; of C22-, C24- and C26-ceramide; and of cysteine. Interestingly, the Odds Ratio of negative patient-reported neuropathy was associated with increased sphingosine.
Plasma levels of the deoxy-sphingoid bases (1-deoxysphinganine, 1-deoxy-sphingosine) were examined as possible predictive biomarkers for type 2 diabetes in a prospective cohort who were followed for 8 years (25). Levels of deoxy-sphingoid bases were elevated in patients with metabolic syndrome, impaired fasting glucose and type 2 diabetes and in patients who developed diabetes during the follow-up period. Deoxy-sphingoid bases levels were found to be significantly elevated in plasma of patients with distal sensorimotor polyneuropathy as confirmed by electroneurographic examinations. Interestingly, the deoxy-sphingoid bases in that study were detectable in early disease stages, but did not correlate with the clinical course (Dohrn et al. 2015). Because in that study deoxysphingosine was analyzed after total hydrolysis of the samples (Dohrn et al. 2015), measured sphingolipid levels can represent both free deoxy-sphingosine and deoxy-ceramides combined. In the type 1 diabetes patients in the current cross-sectional study, levels of DSL correlated negatively with the advanced clinically defined stages of neuropathy (data not shown). Further studies on DSL might lead to a better pathophysiological understanding of diabetic neuropathy.
Little is known concerning the possible role of ceramides in the development of diabetic complications. To our knowledge, this study is the first to demonstrate that plasma levels of select deoxy-ceramide, and ceramide species are associated with the MNSI screening of neuropathy in type 1 diabetes. The association of specific very long chain deoxy-ceramides (C24, C24:1) with neuropathy in the studied DCCT patients is similar to the results from a recent study in breast cancer patients undergoing paclitaxel chemotherapy (Kramer et al. 2015). Paclitaxel is a widely used chemotherapy drug, and one of its major side effects is peripheral neuropathy. It has been found that the incidence and severity of paclitaxel-induced peripheral neuropathy in the breast cancer patients is also associated with the plasma levels of very long chain DSL (Kramer et al. 2015). These results and data from the current study suggest that elevated plasma levels of a specific DSL, such as C24 deoxy-ceramide, might be involved in peripheral neurotoxicity and neuropathy.
In our previous investigations of sphingolipidomics in type 1 diabetes, we determined the association of plasma concentrations of individual ceramide species and the sphingoid bases and their phosphates with the future development of nephropathy in a subgroup of the DCCT/EDIC cohort (Klein et al. 2014). We determined that the concentrations of select ceramide species measured in plasma samples collected at entry into DCCT were significantly lower in patients who developed macroalbuminuria over a period of approximately 15 years (EDIC year 8) than in patients who maintained normal albumin excretion rate. Ceramides are an integral part of cell membrane structure where they can regulate the lateral partitioning of membrane components (Zhang et al. 2009). The formation of ceramide-enriched domains and platforms is largely dependent on the ceramide structure. Properties of ceramides with different fatty acyl chains have been examined in phosphatidylcholine model membranes (Pinto et al. 2011), in animal models (Silva et al. 2012) and in cultured cells in vitro (Hartmann et al. 2013). It was determined that saturated ceramides and very long chain ceramides influence membrane biophysical properties which may compromise cellular processes such as trafficking, sorting and cell proliferation. The exposure of cell membranes to ceramide species with different acyl chain length and saturation might have a distinct biophysical impact on cell signaling and functionality (Pinto et al. 2011; Grösch et al. 2012; Silva et al. 2012; Hartmann et al. 2013; Park et al. 2013). The function of the very long chain deoxy-ceramides is currently unknown. It remains to be investigated in future studies whether these otherwise minor lipid species, when elevated, have a disruptive effect on the membranes of the neurons of the peripheral nervous system ultimately resulting in neurotoxicity.
The mechanism(s) whereby sphingolipid metabolism in the peripheral nervous system may become altered in diabetic patients remains to be determined. The ceramide composition of membranes of cells in the peripheral nervous system may become altered by interacting with plasma lipoproteins, which themselves may contain altered sphingolipid composition. The modifications of lipoproteins frequently associated with diabetes (e.g., glycation and oxidation) may alter the interaction of the lipoprotein with their cell receptors. These changes in cell–lipoprotein interactions may activate cell signaling pathways which then may alter intracellular sphingolipid metabolism. The mechanisms whereby lipoproteins in diabetic patients may alter nerve cell sphingolipid metabolism, and subsequently impact nerve cell physiology, remain to be determined.
In a case-controlled study of 50 patients with type 2 diabetes and a control group of 49 subjects with no history of diabetes, the plasma levels of DSL were significantly elevated in the group of diabetic patients compared to levels observed in the control subjects, while plasma levels of the other normal, physiologic C16 and C18 sphingoid bases did not differ significantly (Bertea et al. 2010). Because plasma DSL levels are also metabolically linked to amino acid metabolism (Dawkins et al. 2001; Penno et al. 2010; Garofalo et al. 2011; Othman et al. 2015), the concentration and distribution of free amino acids in plasma from the diabetic patients and control subjects were also determined in that study (Bertea et al. 2010). Plasma levels of L-serine, the preferred amino acid substrate for the SPT enzyme, were reduced in the diabetics, while the levels of L-alanine, an alternate amino acid substrate used by SPT for DSL formation, were not significantly changed (Bertea et al. 2010). In streptozotocin-induced diabetic rats, L-serine supplementation lowered plasma DSL, likely by outcompeting the L-alanine as substrate for SPT, and improved mechanical sensitivity (Othman et al. 2015). In the present study, plasma levels of L-serine, the preferred substrate for SPT, and of L-alanine and glycine, the alternate substrates for SPT which give rise to DSL, did not differ between the group of patients symptomatic for neuropathy compared to levels in control patients. However, the plasma levels of cysteine were unexpectedly significantly higher in type 1 diabetes patients with neuropathy than those without. The pathophysiological significance of this finding is unclear and remains to be determined.
Conclusions
In conclusion, this pilot study demonstrates that plasma levels of select very long chain deoxy-ceramide and cer-amide species are positively associated with neuropathy in type 1 diabetes and that plasma levels of the amino acid cysteine are elevated in type 1 diabetes patients with neuropathy. Future longitudinal studies will potentially provide more information on the relationship between the clinical severity of neuropathy and DSL as diagnostic and prognostic markers of neuropathy in type 1 diabetes.
Ethical Approval.
The Institutional Review Board (IRB) at the Medical University of South Carolina (MUSC) and all participating DCCT/EDIC centers approved the sample collection procedures. Written informed consent was obtained from all participants.
Acknowledgments
The technical assistance of Ms. Charlyne Chassereau and Ms. Andrea Semler is acknowledged. The authors are grateful to the patients in the DCCT/EDIC for their long-term participation in this important trial.
Funding Financial support for this work was provided by the NIDDK Diabetic Complications Consortium (DiaComp, www.dia comp.org), Grant DK076169 (RLK). This work was also supported by the National Institutes of Health Grant P01-HL55782 (MLV). Additional funding was obtained from the Department of Veterans Affairs Merit Review Program (MLV and RLK). The contents of this manuscript do not represent the views of the Department of Veterans Affairs or the United States Government. Sphingolipid analyses were supported in part by the Lipidomics Shared Resource, Hollings Cancer Center (P30 CA138313), and the Lipidomics Core in the SC Lipidomics and Pathobiology COBRE, Department Biochemistry (P20 RR017677), MUSC. The DCCT/EDIC is sponsored through research contracts from the National Institute of Diabetes, Endocrinology and Metabolic Diseases of the National Institute of Diabetes and Digestive and Kidney diseases (NIDDK) and the National Institutes of Health. Additional support was provided by the National Center for Research Resources through the GCRC program and by Genentech, Inc. through a cooperative research and development agreement with the NIDDK.
Abbreviations
- HSAN1
Hereditary sensory and autonomic neuropathy type 1
- DSL
Deoxy-sphingolipids
- SPT
Serine palmitolytransferase
- DCCT
The Diabetes Control and Complications Trial
- EDIC
Epidemiology of Diabetes Interventions
- AER
Albumin excretion rate
- MNSI
Michigan Neuropathy Screening Instrument
- HPLC-ESI-MS/MS
High-performance liquid chromatography-tandem mass spectrometry
Footnotes
Author Contributions SMH and RLK directed sample analyses and analyzed the data. SMH and RLK wrote the manuscript, and NLB conducted the statistical analyses of the data and wrote the relevant sections. JMELA contributed to data analysis and presentation. SDS, JSP and JB developed the methodology for the deoxy-sphingolipid analysis and generated the sphingolipid data using mass spectroscopy. MFL-V guided patient selection, contributed to the discussion and reviewed/edited the manuscript. SMH and RLK are the guarantors of this work and, as such, had full access to all the data in the study and took the responsibility for the integrity of the data and the accuracy of the data analysis.
Compliance with Ethical Standards
Conflicts of interest The authors declare that they have no conflict of interest.
References
- Baird RD, Kitzen J, Clarke PA, Planting A, Reade S, Reid A, et al. Phase I safety, pharmacokinetic, and pharmacogenomic trial of ES-285, a novel marine cytotoxic agent, administered to adult patients with advanced solid tumors. Molecular Cancer Therapeutics. 2009;8:1430–1437. doi: 10.1158/1535-7163.MCT-08-1167. [DOI] [PubMed] [Google Scholar]
- Bejaoui K, Wu C, Scheffler MD, Haan G, Ashby P, Wu L, et al. SPTLC1 is mutated in hereditary sensory neuropathy, type 1. Nature Genetics. 2001;27:261–262. doi: 10.1038/85817. [DOI] [PubMed] [Google Scholar]
- Bertea M, Rütti MF, Othman A, Marti-Jaun J, Hersberger M, von Eckardstein A, Hornemann T. Deoxysphingoid bases as plasma markers in diabetes mellitus. Lipids in Health and Disease. 2010;9:84. doi: 10.1186/1476-511X-9-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielawski J, Pierce JS, Snider J, Rembiesa B, Szulc ZM, Bielawska A. Comprehensive quantitative analysis of bioactive sphingolipids by high-performance liquid chromatography-tandem mass spectrometry. Methods in Molecular Biology. 2009;579:443–467. doi: 10.1007/978-1-60761-322-0_22. [DOI] [PubMed] [Google Scholar]
- Dawkins JL, Hulme DJ, Brahmbhatt SB, Auer-Grumbach M, Nicholson GA. Mutations in SPTLC1, encoding serine palmitoyltransferase, long chain base subunit-1, cause hereditary sensory neuropathy type I. Nature Genetics. 2001;27:309–312. doi: 10.1038/85879. [DOI] [PubMed] [Google Scholar]
- Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. New England Journal of Medicine. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- Dohrn MF, Othman A, Hirshman SK, Bode H, Alecu I, Fähndrich E, et al. Elevation of plasma 1-deoxysphingolipids in type 2 diabetes mellitus: A susceptibility to neuropathy? European Journal of Neurology. 2015;22:806–814. doi: 10.1111/ene.12663. [DOI] [PubMed] [Google Scholar]
- Duan J, Merrill AH., Jr 1-Deoxysphingolipids encountered exogenously and made de novo: Dangerous mysteries inside an enigma. Journal of Biological Chemistry. 2015;290:15380–15389. doi: 10.1074/jbc.R115.658823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyck PJ, Karnes J, O'Brien PC, Swanson CJ. Neuropathy Symptom Profile in health, motor neuron disease, diabetic neuropathy, and amyloidosis. Neurology. 1986;36:1300–1308. doi: 10.1212/wnl.36.10.1300. [DOI] [PubMed] [Google Scholar]
- Epidemiology of Diabetes Interventions and Complication (EDIC) Research Group. Epidemiology of Diabetes Interventions and Complications (EDIC). Design, implementation, and preliminary results of a long-term follow-up of the Diabetes Control and Complications Trial cohort. Diabetes Care. 1999;22:99–111. doi: 10.2337/diacare.22.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman EL, Stevens MJ, Thomas PK, Brown MB, Canal N, Greene DA. A practical two-step quantitative clinical and electrophysiological assessment for the diagnosis and staging of diabetic neuropathy. Diabetes Care. 1994;17:1281–1289. doi: 10.2337/diacare.17.11.1281. [DOI] [PubMed] [Google Scholar]
- Fox TE, Kester M. Therapeutic strategies for diabetes and complications: A role for sphingolipids? Advances in Experimental Medicine and Biology. 2010;688:206–216. doi: 10.1007/978-1-4419-6741-1_14. [DOI] [PubMed] [Google Scholar]
- Galadari S, Rahman A, Pallichankandy S, Galadari A, Thayyullathil F. Role of ceramide in diabetes mellitus: Evidence and mechanisms. Lipids in Health and Disease. 2013;12:98. doi: 10.1186/1476-511X-12-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garofalo K, Penno A, Schmidt BP, Lee HJ, Frosch MP, von Eckardstein A. Oral L-serine supplementation reduces production of neurotoxic deoxysphingolipids in mice and humans with hereditary sensory autonomic neuropathy type 1. Journal of Clinical Investigation. 2011;121:4735–4745. doi: 10.1172/JCI57549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grösch S, Schiffmann S, Geisslinger G. Chain length-specific properties of ceramides. Progress in Lipid Research. 2012;51:50–62. doi: 10.1016/j.plipres.2011.11.001. [DOI] [PubMed] [Google Scholar]
- Hammad SM, Pierce JS, Soodavar F, Smith KJ, Al Gadban MM, Rembiesa B, et al. Blood sphingolipidomics in healthy humans: Impact of sample collection methodology. Journal of Lipid Research. 2010;51:3074–3087. doi: 10.1194/jlr.D008532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannun YA, Obeid LM. Principles of bioactive lipid signalling: Lessons from sphingolipids. Nature Review Molecular Cell Biology. 2008;9:139–150. doi: 10.1038/nrm2329. [DOI] [PubMed] [Google Scholar]
- Hartmann D, Wegner MS, Wanger RA, Ferreirós N, Schreiber Y, Lucks J, et al. The equilibrium between long and very long chain ceramides is important for the fate of the cell and can be influenced by co-expression of CerS. International Journal of Biochemistry & Cell Biology. 2013;45:1195–1203. doi: 10.1016/j.biocel.2013.03.012. [DOI] [PubMed] [Google Scholar]
- Herman WH, Pop-Busui R, Braffett BH, Martin CL, Cleary PA, Albers JW, et al. Use of the Michigan Neuropathy Screening Instrument as a measure of distal symmetrical peripheral neuropathy in Type 1 diabetes: Results from the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications. Diabetic Medicine. 2012;29:937–944. doi: 10.1111/j.1464-5491.2012.03644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein RL, Hammad SM, Baker NL, Hunt KJ, Al Gadban MM, Cleary PA, et al. Decreased plasma levels of select very long chain ceramide species are associated with the development of nephropathy in type 1 diabetes. Metabolism. 2014;63:1287–1295. doi: 10.1016/j.metabol.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer R, Bielawski J, Kistner-Griffin E, Othman A, Alecu I, Ernst D, et al. Neurotoxic 1-deoxysphingolipids and paclitaxel-induced peripheral neuropathy. FASEB Journal. 2015;29:4461–4472. doi: 10.1096/fj.15-272567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massard C, Salazar R, Armand JP, Majem M, Deutsch E, García M, et al. Phase I dose-escalating study of ES-285 given as a three-hour intravenous infusion every three weeks in patients with advanced malignant solid tumors. Investigational New Drugs. 2012;30:2318–2326. doi: 10.1007/s10637-011-9772-8. [DOI] [PubMed] [Google Scholar]
- Othman A, Bianchi R, Alecu I, Wei Y, Porretta-Serapiglia C, Lombardi R, et al. Lowering plasma 1-deoxysphingolipids improves neuropathy in diabetic rats. Diabetes. 2015;64:1035–1045. doi: 10.2337/db14-1325. [DOI] [PubMed] [Google Scholar]
- Othman A, Rütti MF, Ernst D, Saely CH, Rein P, Drexel H, et al. Plasma deoxysphingolipids: A novel class of biomarkers for the metabolic syndrome? Diabetologia. 2012;55:421–431. doi: 10.1007/s00125-011-2384-1. [DOI] [PubMed] [Google Scholar]
- Park JW, Park WJ, Kuperman Y, Boura-Halfon S, Pewzner-Jung Y, Futerman AH. Ablation of very long acyl chain sphingolipids causes hepatic insulin resistance in mice due to altered detergent-resistant membranes. Hepatology. 2013;57:525–532. doi: 10.1002/hep.26015. [DOI] [PubMed] [Google Scholar]
- Penno A, Reilly MM, Houlden H, Laurá M, Rentsch K, Niederkofler V, et al. Hereditary sensory neuropathy type 1 is caused by the accumulation of two neurotoxic sphingolipids. Journal of Biological Chemistry. 2010;285:11178–11187. doi: 10.1074/jbc.M109.092973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto SN, Silva LC, Futerman AH, Prieto M. Effect of ceramide structure on membrane biophysical properties: The role of acyl chain length and unsaturation. Biochimica Biophysica Acta. 2011;1808:2753–2760. doi: 10.1016/j.bbamem.2011.07.023. [DOI] [PubMed] [Google Scholar]
- Rotthier A, Auer-Grumbach M, Janssens K, Baets J, Penno A, Almeida-Souza L, et al. Mutations in the SPTLC2 subunit of serine palmitoyltransferase cause hereditary sensory and autonomic neuropathy type I. American Journal of Human Genetics. 2010;87:513–522. doi: 10.1016/j.ajhg.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schöffski P, Dumez H, Ruijter R, Miguel-Lillo B, Soto-Matos A, Alfaro V, Giaccone G. Spisulosine (ES-285) given as a weekly three-hour intravenous infusion: Results of a phase I dose-escalating study in patients with advanced solid malignancies. Cancer Chemotherapy and Pharmacology. 2011;68:1397–1403. doi: 10.1007/s00280-011-1612-1. [DOI] [PubMed] [Google Scholar]
- Silva LC, Ben David O, Pewzner-Jung Y, Laviad EL, Stiban J, Bandyopadhyay S, et al. Ablation of ceramide synthase 2 strongly affects biophysical properties of membranes. Journal of Lipid Research. 2012;53:430–436. doi: 10.1194/jlr.M022715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei N, Pan J, Pop-Busui R, Othman A, Alecu I, Hornemann T, Eichler FS. Altered sphingoid base profiles in type 1 compared to type 2 diabetes. Lipids in Health and Disease. 2014;13:161. doi: 10.1186/1476-511X-13-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Li X, Becker KA, Gulbins E. Ceramide-enriched membrane domains-structure and function. Biochimca Biophysica Acta. 2009;1788:178–183. doi: 10.1016/j.bbamem.2008.07.030. [DOI] [PubMed] [Google Scholar]
- Zheng W, Kollmeyer J, Symolon H, Momin A, Munter E, Wang E, et al. Ceramides and other bioactive sphingolipid backbones in health and disease: Lipidomic analysis, metabolism and roles in membrane structure, dynamics, signaling and autophagy. Biochimica Biophysica Acta. 2006;1758:1864–1884. doi: 10.1016/j.bbamem.2006.08.009. [DOI] [PubMed] [Google Scholar]