Scheme 2.
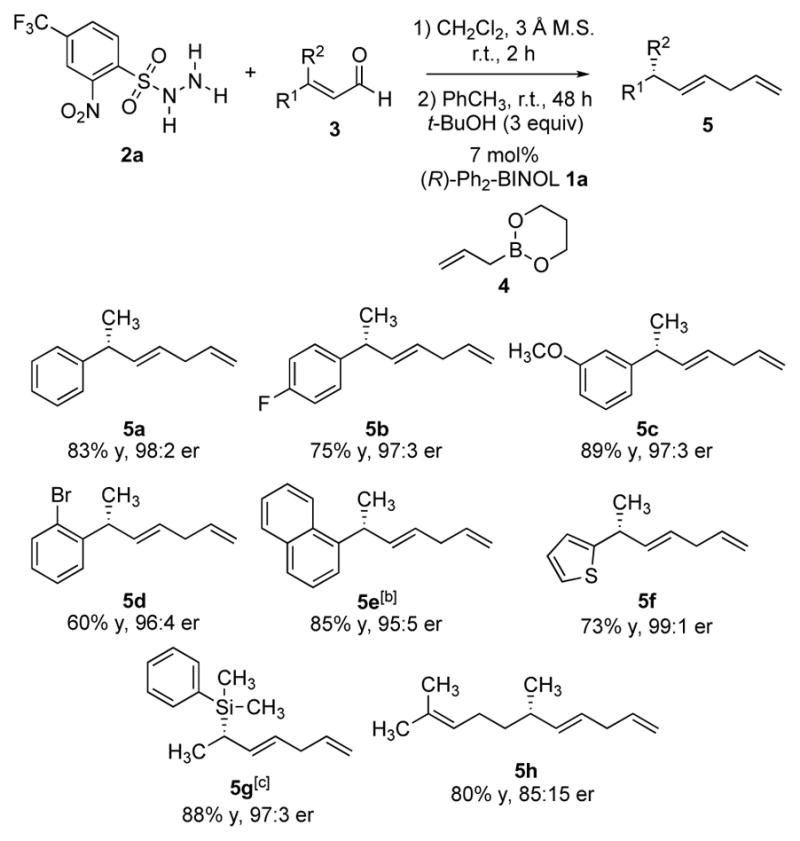
Enantioselective reductive allylation of β-methyl enals.[a] [a] All reactions were run on 0.4 mmol scale. Yields of isolated products are given. The e.r. values were determined by HPLC analysis using chiral stationary phases. [b] Reaction was run at 1 M concentration. [c] 5 equivalents of t-BuOH were used in the absence of toluene. The (Z)-silyl enal 3g was used in the reaction. See Supplementary Information for experimental details and stereochemical proof.
