Summary
The premotor cortex (PM) receives inputs from parietal cortical areas representing processed visuospatial information, translates that information into programs for particular movements, and communicates those programs to the primary motor cortex (M1) for execution. Consistent with this general function, intracortical microstimulation (ICMS) in PM of sufficient frequency, amplitude, and duration has been shown to evoke complex movements of the arm and hand that vary systematically depending on the locus of stimulation. Using frequencies and amplitudes too low to evoke muscle activity, however, we found that ICMS in PM can provide instructions to perform specific reach, grasp, and manipulate movements. These instructed actions were not fixed, but rather were learned through associations between the arbitrary stimulation locations and particular movements. Low-amplitude ICMS at different PM locations thus evokes distinguishable experiences that can become associated with specific movements arbitrarily, providing a novel means of injecting information into the nervous system.
INTRODUCTION
In reaching to grasp an object, the premotor cortex (PM) commonly is thought to translate visuospatial information received from the posterior parietal cortex into motor plans specific to the object’s location and shape (Grafton, 2010; Karl and Whishaw, 2013). PM also may participate in associating instructional cues with particular movements. For example, PM neurons modulate differently when movements are executed based on external visual instructions versus internal memory-based guidance (Mushiake et al., 1991), and inactivation of dorsal PM (PMd) selectively impairs performance under internal guidance (Ohbayashi et al., 2016). The activity of PMd neurons also has been shown to change as monkeys learn arbitrary associations between particular visual (Mitz et al., 1991) or auditory (Germain and Lamarre, 1993) instructions and specific movements.
Consistent with the role of PM in formulating motor plans for reaching to a particular location with a hand shape appropriate for grasping a target object, conventional short trains of intracortical microstimulation (ICMS) in PM can elicit twitches in upper extremity musculature (Gentilucci et al., 1988; Rizzolatti et al., 1988; Weinrich and Wise, 1982). Moreover, long-duration ICMS at different loci in macaque PM or in the primary motor cortex (M1) can drive complex movements of the entire upper extremity to particular locations and distinct hand shapes (Graziano, 2016; Graziano et al., 2002; Griffin et al., 2014; Overduin et al., 2012). The particular movements evoked by long-duration ICMS in PM and M1 collectively have been found to be clustered spatially into maps of various ethologically relevant movements (Graziano, 2016; Graziano et al., 2002). Similar observations have been made in other cortical areas including the posterior parietal cortex, as well as in other species including humans (Baldwin et al., 2017; Desmurget et al., 2014; Stepniewska et al., 2005). In human parietal cortex, upon intraoperative stimulation of the cortical surface, subjects reported an intention to make a particular movement or described an experience that a particular movement had been made even though no movement or electromyographic (EMG) activity occurred; however, similar stimulation of PM evoked actual movements that the subject denied having made (Desmurget et al., 2009). Taken together, these findings suggest that electrical activation of particular loci in PM leads to the generation of specific movements without eliciting experiences of which the subject is aware.
Other studies suggest, however, that subjects may be aware of PM activation. In early studies involving electrical stimulation of the cortical surface of the precentral gyrus (including PM), humans occasionally did report a desire to move a particular body part, though no movement actually occurred (Penfield and Rasmussen, 1950). Similarly in the human supplementary motor area (SMA), stimulation of the cortical surface occasionally evoked an urge to move or a tingling sensation (Fried et al., 1991). Indeed, some neurons in macaque ventral PM (PMv) respond to vibrotactile stimuli on a fingertip with firing rates that depend on the vibratory frequency (Romo et al., 2004). Other PMv neurons have large and interacting somatosensory and visual receptive fields (Graziano, 1999; Rizzolatti et al., 1981a, b). These observations raise the possibility that low-amplitude ICMS at different PM loci, without directly driving a movement, might nevertheless elicit sensory percepts, desires to perform different movements, or other thoughts of which the subject becomes to some extent “aware.” If activation of different sites in PM elicits a variety of experiences of which the subject is aware, can the subject report different experiences by learning to associate each experience arbitrarily with the generation of a specific movement?
Here, we tested the hypothesis that the association between activation of particular PM loci and the generation of specific movements is not based on a fixed mapping, but rather can be learned. We activated PM loci with ICMS at current amplitudes too low to evoke muscle activity or drive movements. We reasoned that if such low-amplitude ICMS at different PM loci only biased the unaware subject subliminally, then the subject would be unable to use ICMS at different PM loci as instructions for executing arbitrarily-assigned movements. But if low-amplitude ICMS evoked distinguishable experiences of which the subject was aware, then the subject could learn to use these experiences as instructions to perform specific, arbitrarily-associated movements.
In monkeys previously trained to perform a reach-grasp-manipulate (RGM) task, we delivered low-amplitude ICMS at arbitrary PM locations concurrently with visual instruction cues. We found that when the visual instruction cues were gradually dimmed, the monkeys learned to associate even brief, low-frequency, PM-ICMS instructions with specific actions. Furthermore, after the assignments of PM loci to instruct particular RGM movements were shuffled, the monkeys re-learned the shuffled assignments, indicating that the arbitrary associations were learned, not fixed. Our findings show that low-amplitude, low-frequency, short-duration PM-ICMS at different loci produces experiences that the subject can distinguish and learn to use as instructions for performing arbitrarily associated movements.
RESULTS
Learning to Use ICMS Instructions
We studied two monkeys previously trained to turn a sphere, push a button, pull a coaxial cylinder, or pull a perpendicular cylinder, instructed by illumination of different light emitting diodes (LEDs) indicating the correct target object on each trial (Figure 1A). Each monkey also had been implanted with multiple 16-channel microelectrode arrays in PM, M1, and the primary somatosensory cortex (S1) (Figure 1D,E). ICMS in S1 (S1-ICMS) can generate percepts comparable to normal vibrotactile sensations (Flesher et al., 2016; Romo et al., 1998; Tabot et al., 2013) and has been used by others to instruct animals which of two actions to perform (Fitzsimmons et al., 2007; O’Doherty et al., 2009). Therefore, we first trained monkey L to perform the four RGM movements using S1-ICMS instructions. Different sets of S1 electrodes and different periodic pulse frequencies were assigned arbitrarily to each object (Table S1). Initially (Figure 2A, solid vertical line), the assigned S1-ICMS and LED instructions were delivered concurrently. After 19 sessions (Figure 2A, dotted vertical line), the LEDs were dimmed gradually over several sessions, initially prolonging reaction time and eventually reducing success rate. After additional dimming of the LEDs, success rate improved progressively as reaction time shortened and became less variable. Eventually the LEDs were turned off entirely (Figure 2A, dashed vertical line), and monkey L continued to perform well using only S1-ICMS instructions. Success rates and reaction times gradually returned to levels comparable to those observed initially, when LED instructions had been available.
Figure 1.
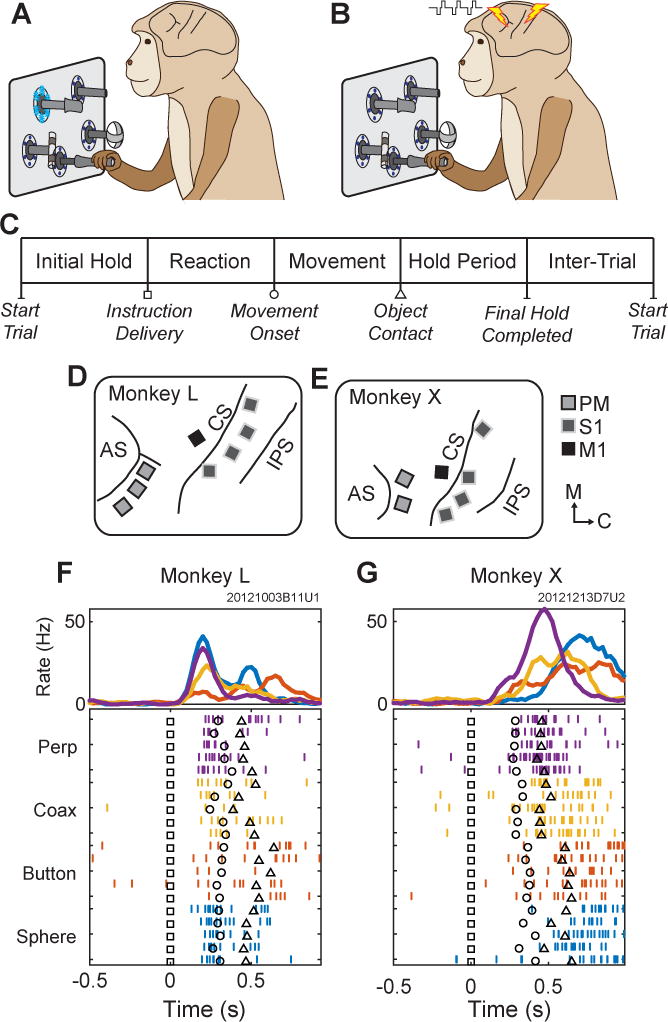
Task, instructions, and electrode locations. (A) Monkeys initially performed the reach-grasp-manipulate task instructed by blue LEDs. (B) Monkeys then learned to perform the same task instructed by ICMS delivered in either PM or S1. (C) Sequence of task epochs. (D,E) Floating microelectrode array locations in (D) monkey L and (E) monkey X. Light gray squares with dark outlines represent arrays in PM; dark gray squares with light outlines, S1, and black squares, M1. AS-arcuate sulcus, CS-central sulcus, IPS-intraparietal sulcus. Orientation arrows: M–medial, C–caudal. (F,G) Typical unit activity recorded from a PM electrode in each monkey during RGM movements (LED Instructions) involving each object: perpendicular cylinder (Perp, purple); coaxial cylinder (Coax, yellow); button (red); and sphere (blue). Only five trials of each movement are shown in the raster display, whereas ~20 trials of each are averaged in the histograms. Trials have been aligned at the instruction onset (Time=0, black squares in the raster trials). Additional markers indicate the time of movement onset (black circle) and target object contact (black triangle) in each trial.
Figure 2.
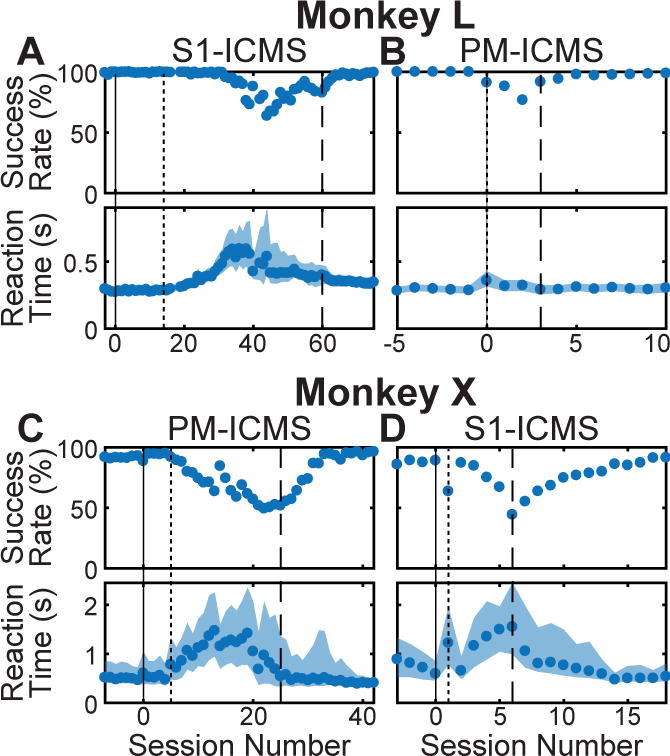
Learning to use PM- or S1-ICMS instructions. In early sessions, only LED instructions were delivered. Beginning at session 0 (solid vertical line), both LED and ICMS instructions were delivered concurrently. Instruction LEDs then were gradually dimmed (beginning at dotted vertical line) and eventually were turned off completely (dashed vertical line). Monkey L first learned to use (A) S1-ICMS then (B) PM-ICMS. Monkey X first learned to use (C) PM-ICMS then (D) S1-ICMS. Reaction times are displayed as medians. The shaded regions represent the 25th to 75th percentile. See also Tables S1 and S1.
Next, we trained monkey L to use instructions delivered with ICMS in PM (PM-ICMS). Different PM-ICMS instructions were paired arbitrarily with the LED instructions. Monkey L learned to use PM-ICMS instructions quickly (Figure 2B). Was prior experience with S1-ICMS needed for the monkey to learn to use PM-ICMS instructions? To address this question, we trained another monkey, monkey X, to use PM-ICMS first. Without any exposure to S1-ICMS instructions, monkey X learned to use PM-ICMS instructions at high success rates (Figure 2C). Thereafter we trained monkey X to use S1-ICMS instructions (Figure 2D).
Once each monkey had learned to use PM-ICMS, we performed a few additional sessions (3 for monkey L, 4 for monkey X) in which certain trials started with particular target objects assigned by the task-control system, but no ICMS was delivered (nor were instructions delivered with LEDs). If the monkey performed a movement correctly to the assigned target, a reward was given. These no-ICMS catch trials occurred randomly every 15–19 trials for monkey L and every 15–20 trials for monkey X, and had no fixed association with any particular target object. Averaged across these sessions, monkey L had a success rate of 99% on trials instructed with PM-ICMS but only 19% (chance = 25%) on no-ICMS catch trials; monkey X had a success rate of 96% on trials instructed with PM-ICMS but only 9% on no-ICMS catch trials. These observations confirm that both monkeys used the PM-ICMS instructions to perform the task.
Comparing Performance with PM-ICMS, S1-ICMS, or LED Instructions
Once each monkey had become proficient with a given type of ICMS instruction, we conducted four consecutive sessions during which those ICMS instructions and LED instructions were interleaved pseudorandomly on different trials (Movies S1, S2). Success rates, reaction times, and movement times were generally similar whether the monkey was instructed using PM-ICMS, S1-ICMS, or LED instructions; however, some differences were identified when data was pooled across the 8 sessions for each monkey (Table S2). Both monkeys achieved higher success rates with either PM-ICMS or LED instructions than with S1-ICMS instructions (p < 0.0001, X2 tests, Bonferroni post-hoc tests). Both monkeys also reacted faster to PM-ICMS than to S1-ICMS or LED instructions (p < 0.0001, Kruskal-Wallis tests, Tukey post-hoc tests). Monkey L moved faster with PM-ICMS instructions than with either S1-ICMS or LED instructions, although monkey X moved fastest with S1-ICMS instructions (p < 0.0001, Kruskal-Wallis tests, Tukey post-hoc tests). Performance thus tended to be slightly better with PM-ICMS than with S1-ICMS or LED instructions. Note that we empirically used different numbers of electrodes, different currents per electrode, and different frequency ranges for PM-ICMS and S1-ICMS in each monkey. Nevertheless, our observation that performance tended to have been slightly but significantly better on average with PM-ICMS than with S1-ICMS was unexpected given that PM-ICMS had been delivered with, if anything, fewer electrodes, lower current per electrode, and narrower frequency ranges, than S1-ICMS (Table S1).
How Much Stimulation was Necessary?
Trains of high-frequency, long-duration (500 ms) ICMS in PM or in M1 have been shown to drive the arm and hand to particular configurations (Graziano et al., 2002; Griffin et al., 2014). As seen in the supplemental movies, (movies S1 and S2), however, our monkeys performed essentially the same voluntary RGM movements whether instructed with LEDs or with PM-ICMS. We therefore sought to determine the minimal pulse frequency, current amplitude and train duration needed for the monkeys to perform well with PM-ICMS instructions.
To do so, we evaluated performance as one of the three parameters was varied (swept) while the other two parameters were held constant (Table S3). When sweeping frequency, the same periodic frequency was used for all four instructions. Both monkeys performed well using stimulation frequencies of 100, 70, or 50 Hz, below which success rates declined and reaction times increased, whereas movement times showed little change (Figure 3A–C). That both monkeys performed well using the same frequency (if greater than 50 Hz) for all four instructions indicated that the monkeys relied not on frequency but rather on the particular electrodes stimulated.
Figure 3.
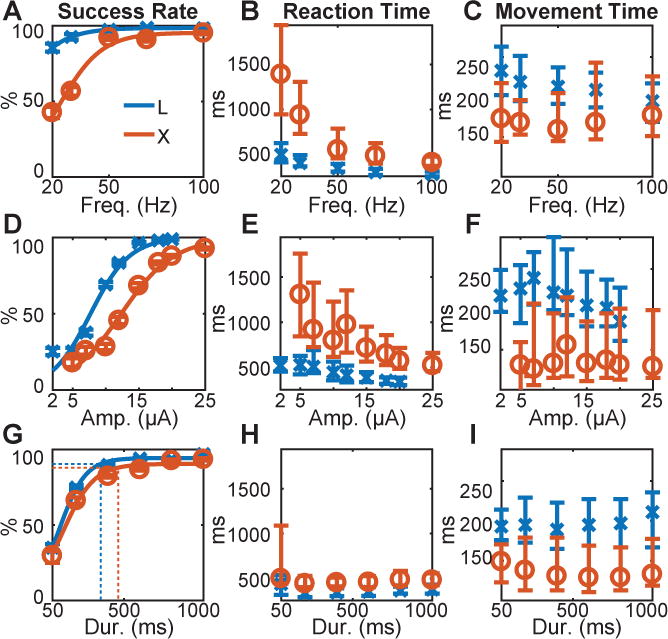
Parameter sweeps. Three parameters of PM-ICMS instructions were varied for each monkey: (A,B,C) pulse frequency, (D,E,F) current amplitude, and (G,H,I) train duration. Success rate (A,D,G), reaction time (B,E,H), and movement time (C,F,I), each have been plotted as a function of the swept parameter. In (G) the vertical dotted lines represent the median reaction time across all swept durations for each monkey (H), and the corresponding horizontal dotted lines indicate the success rate at this median reaction time, based on the fitted logistic functions. X’s (monkey L, blue) and O’s (monkey X, red) indicate medians; error bars represent the 25th and 75th percentiles. See also Table S3.
We therefore made two additional changes. First, we eliminated any periodic frequency by delivering each train with inter-pulse intervals chosen randomly from a uniform distribution ranging from 12.5 to 40 ms (mean 26.25 ms) for monkey L, and from 12.5 to 30 ms (mean 21.25 ms) for monkey X (Table S4). Second, we dropped electrodes from the set used for each instruction until each of the four instructions was delivered through a different, single, PM electrode. Both monkeys continued to perform well. We then swept pulse amplitudes and train durations using these stochastically jittered pulse trains delivered through a different single electrode for each instruction. Both monkeys performed greater than 80% of trials correctly with amplitudes down to 18 μA, and with train durations down to 400 ms, below which success rates rolled off (Figure 3D,G). Reaction time increased progressively as amplitude was reduced, whereas shortening the train duration had little effect (Figure 3E,H). Notably, as the train duration became shorter than the median reaction times (Figure 3G, vertical dotted lines), both monkeys continued to perform substantially above chance (25%) even though the stimulation often had ended before movement onset. At a train duration of 200 ms, monkey L performed 74.8% of trials correctly with a median reaction time of 336 ms while PM-ICMS instructions consisted of an average of 7.5 jittered pulses through a single electrode (10–28 μA; 26.5 ms mean interval); monkey X performed 66.1% of trials correctly with a median reaction time of 443 ms while PM-ICMS instructions consisted of an average of 9.4 jittered pulses through a single electrode (20–25 μA; 21.25 ms mean interval). In contrast to success rates and reaction times, movement times remained relatively unaffected by reductions of frequency, amplitude, or train duration (Figure 3C,F,I), indicating PM-ICMS did not drive movements directly but instead instructed the monkey which movement to perform.
PM-ICMS Instructions Did Not Activate Muscles
In M1, ICMS can evoke muscle twitches at currents below 40 μA, whereas currents above 60 μA generally are needed in PM (Gentilucci et al., 1988; Kwan et al., 1978; Weinrich and Wise, 1982). Although we used currents below 30 μA for PM-ICMS, we considered the possibility that PM-ICMS might have evoked twitches in muscles that the monkeys felt and used as instructions. We therefore applied stimulus-triggered averaging (StimTA) of rectified EMG as a sensitive method for detecting low-amplitude, short-latency muscle activation output effects evoked by ICMS (Park et al., 2004).
Given that units related to RGM movements had been recorded from these PM electrodes during the LED-instructed task (Fig 1F,G), in selected sessions we recorded EMG activity from four upper extremity muscle groups (biceps, triceps, forearm flexors, and forearm extensors) as the monkeys performed the RGM movements instructed by PM-ICMS on different, single electrodes. In intermixed catch-trials occurring every 8–15 trials, we instead delivered trains of jittered ICMS through a single electrode in M1 (M1-ICMS) in place of a pseudorandomly selected PM-ICMS instruction. M1-ICMS catch-trials had no fixed association with particular target objects (catch-trial success rate for monkey L, 22.1%; monkey X, 10.6%). In each session (2 for monkey L, 4 for monkey X), catch-trial M1-ICMS was delivered through a different M1 electrode while consistently using the same four single electrodes in PM to deliver instructions. Identical current amplitudes and inter-pulse interval distributions were used for PM-ICMS and M1-ICMS in each monkey (Table S4).
Figure 4 shows post-stimulus effects (PStEs) from one such session for each monkey. ICMS pulses delivered through the M1 electrode during catch trials produced PStEs in one muscle group in monkey L and in three muscle groups in monkey X (green traces). In contrast, no significant PStEs were evoked by the pulses delivered through any of the four PM electrodes used to instruct the four objects. Overall, catch-trial ICMS at a different M1 electrode for each session produced PStEs in 1 of the 2 sessions in monkey L and in 2 of the 4 sessions in monkey X. In none of the 6 sessions did PM-ICMS at any of the four electrodes produce a significant PStE in the arm or forearm muscles of either monkey. The paucity of muscle activation evoked by ICMS at the PM electrodes indicates the monkeys were unlikely to have felt muscle twitches evoked by PM-ICMS that they used as instructions.
Figure 4.
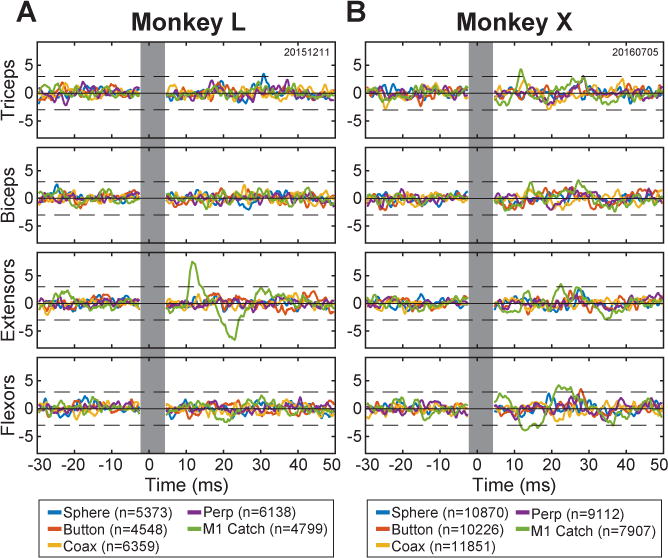
Stimulus-triggered averages of EMG activity. Averages of rectified EMG activity from four upper extremity muscle groups—Triceps, Biceps, forearm Extensors, and forearm Flexors—were compiled for each monkey (A, monkey L; B, monkey X) using individual ICMS pulses as triggers. ICMS parameters used for these sessions are given in Table S4. Separate averages (colors) were compiled using n pulses delivered through the PM electrode instructing the sphere, button, coaxial cylinder, or perpendicular cylinder, as well as for catch-trial pulses delivered in M1. The vertical gray bar indicates the interval during which the aligned stimulation artifacts have been blanked. Each trace has been z-scored. The dashed horizontal black lines represent ± 3 standard deviations from the bootstrap-adjusted baseline, i.e. the solid black horizontal line. See also Table S4 and Figure S1.
After all other studies had been completed, we expanded the number of muscles from which we recorded (now including head and shoulder muscles), and used higher current amplitudes to determine if any output effects could be produced from the electrodes used for PM-ICMS instructions. In monkey L, no PStEs were produced from any of the PM electrodes at currents up to 55 μA in either of 2 sessions (Figure S1A). In monkey X, no PStEs were produced from 3 of the 4 PM electrodes (Figure S1B). The fourth electrode (yellow trace, coax trials) consistently produced PStEs in the post-auricular and frontalis muscles, including a weak PStE in the frontalis muscle at the current used for PM-ICMS instructions (25 μA). The absence of output effects from some electrodes was not unexpected, given that the tips of our implanted electrodes were positioned randomly with respect to the cortical layers, and hence were not necessarily close to layers where ICMS evokes output effects (Andersen et al., 1975; Park et al., 2004).
Given that all 4 PM electrodes in monkey L and 3 of the 4 in monkey X failed to produce PStEs at currents up to 55 μA, we also applied more potent, conventional, short trains of 333 Hz ICMS to look for output effects from electrodes that did not produce PStEs (Park et al., 2004). While delivering such conventional ICMS trains randomly every 1–4 seconds, we observed the tranquilized monkey for twitches of the face, upper extremity, trunk, or lower extremity. In monkey L, no twitches were evoked by delivering short-train ICMS at currents up to 100 μA at any of the 4 PM electrodes. In monkey X, a twitch of the right pinna was evoked from 1 PM electrode (different from the electrode that produced PStEs). In comparison, the same short-train ICMS evoked clear twitches in arm and/or forearm muscles when delivered at each of the M1 electrodes used for M1-ICMS (2 electrodes in monkey L; 4 in monkey X). We cannot rule out the possibility that weak twitches evoked in the frontalis muscle by ICMS pulses delivered through one PM electrode might have been felt by monkey X and used as the instruction for one target object. We found no evidence, however, of output effects from low-amplitude ICMS delivered through the other PM electrodes in monkey X or from any of the PM electrodes in monkey L that would have enabled either monkey to perform the four different movements at the observed success rates of > 90%.
Re-learning Conditional Associations
Although PM-ICMS instructions had been assigned to different electrodes arbitrarily, we considered the possibility that we had picked loci at which PM-ICMS fortuitously biased the movement to each of the arbitrarily assigned objects (Graziano et al., 2002). We therefore retrained each monkey to use the same single-electrode, jittered, PM-ICMS trains, but reassigned each instruction electrode to a changed target object. Both monkeys learned to use the reassigned instructions, achieving high success rates (91.4% for monkey L, 93.8% for monkey X). That the monkeys re-learned to use identical PM-ICMS trains delivered to the same single electrode as instructions for movements involving a different object at a different location indicates that our PM-ICMS did not drive movements to particular locations or objects. Rather, we infer that the monkeys first learned and then re-learned conditional associations between each PM-ICMS instruction (i.e. ICMS at a particular electrode) and a particular movement.
DISCUSSION
We found that low-amplitude microstimulation delivered at arbitrary loci in premotor cortex can provide instructions to perform particular movements. PM-ICMS remained effective in providing instructions even though the ICMS we delivered was too low in amplitude and too short in duration to drive the arm and hand into specific configurations, or even to evoke muscle activation. Furthermore, the association between PM-ICMS at a particular electrode with the execution of a particular RGM movement could be relearned when the mapping of four PM electrodes to the four RGM movements was shuffled, indicating that PM-ICMS at a given site did not evoke a fixed movement. Rather, the association between PM-ICMS at a given site and execution of a particular RGM movement was learned (Germain and Lamarre, 1993; Mitz et al., 1991).
ICMS in S1 is known to evoke percepts comparable to natural tactile stimuli at somatic locations topographically appropriate for the electrode location (Flesher et al., 2016; Romo et al., 1998). We infer that the association between such percepts evoked by the present S1-ICMS instructions and execution of particular RGM movements likewise was learned. With multi-electrode instructions, both monkeys had slightly faster reaction times and slightly higher success rates when using PM-ICMS instructions than when using S1-ICMS instructions, although PM-ICMS had involved, if anything, fewer electrodes, lower current amplitude per electrode, and narrower frequency ranges. Moreover, the PM-ICMS trains we used to inject instructions were generally lower in amplitude and frequency than the ICMS used in prior studies of S1 to produce artificial sensations or provide feedback (Dadarlat et al., 2015; Romo et al., 1998; Tabot et al., 2013).
While unanticipated, these differences are supported further by comparing the present studies using PM-ICMS to instruct our four-alternative task (chance = 25%) with prior studies that assessed ICMS detection thresholds in macaque S1 using two-alternative forced-choice tasks (chance = 50%) (Kim et al., 2015a; Kim et al., 2015b). With respect to frequency, the present monkeys both performed at success rates > 80% using PM-ICMS down to 50 Hz (Figure 3A, stimulating at < 35 μA, adjusted up from 30 μA for use of 3–4 electrodes simultaneously (Kim et al., 2015b, Figure 2) in the present frequency sweeps), whereas ICMS in S1 was detected on < 70% of trials at 50 Hz (Kim et al. 2015a, Figure 2b, 40 μA). With respect to current, the present monkeys both performed at success rates > 80% using PM-ICMS down to currents of 18 μA (Figure 3D, jittered ICMS with minimum inter-pulse intervals of 12.5 ms corresponding to a maximal frequency of 80 Hz), whereas ICMS in S1 was detected on < 70 % of trials using 30 μA currents (at 100 Hz, Kim et al., 2015a, Figure 2b). With respect to train duration, the present monkeys performed at success rates > 80 % using PM-ICMS train durations down to 400 ms and > 65% down to 200 ms (Figure 3G, jittered ICMS at currents < 30 μA), whereas ICMS in S1 was detected on < 70% of trials using train durations of 500 ms down to 200 ms (Kim et al., 2015a, Figure 2c, 300 Hz at 30 μA current). Overall, ICMS thus may be experienced more readily in PM than in S1.
Our studies did not address the nature of the experiences evoked by PM-ICMS. Although we selected PM electrodes based on evidence that they were situated in active cortex (spiking activity recorded during RGM movements), our randomly jittered PM-ICMS trains were unlikely to have mimicked the natural activity of any local neurons. Nevertheless, as suggested in the Introduction, we can speculate that the low-amplitude, jittered PM-ICMS trains we used may have evoked somatosensory and/or visual percepts, desires to move particular body parts, or other internal urges or thoughts, any of which the monkeys could have used as instructions. Anterior to PM, the SMA (Fried et al., 1991), and the frontal eye fields (Murphey and Maunsell, 2008), and superior to the inferior frontal language areas, electrical stimulation in other frontal cortical areas generally has not been thought to evoke experiences—sensory percepts, feelings, urges, thoughts, etc.—of which the subject is aware (Desmurget et al., 2009; Penfield and Rasmussen, 1950). In future studies, the experimental paradigm we used here could be applied to determine whether or not low-amplitude ICMS gives rise to experiences of which the subject is aware when delivered in other frontal areas including the pre-SMA, dorsal or ventral prefrontal cortex, and anterior cingulate cortex.
Whereas a number of recent studies have shown that ICMS in S1 or in the primary visual cortex can deliver sensory information (Flesher et al., 2016; Lewis et al., 2015; Romo et al., 1998; Tabot et al., 2013; Troyk et al., 2003), our results demonstrate that ICMS can be used to inject arbitrary, task-specifying instructions into PM, an association cortical area. A learning period was required for our subjects to respond reliably to stimulation at particular electrodes with particular movements, not entirely unlike the period during which human subjects learn to interpret the input from a cochlear implant (Fu and Galvin, 2007). If future studies identify additional cortical association areas in which ICMS can deliver information that subjects can learn to interpret, the potential territory in which ICMS might be applied to transmit information into the nervous system may expand considerably. Artificial electrical stimulation of muscles based on information decoded from M1 already is being developed to bridge spinal cord injuries with cortico-muscular neuroprosthetic devices, thereby restoring lost movement abilities (Bouton et al., 2016; Capogrosso et al., 2016; Ethier et al., 2012). The capability of injecting interpretable information arbitrarily into association cortical areas may enable the development of cortico-cortical neuroprosthetic devices that bridge across injured brain regions by decoding neural signals from upstream areas and injecting the processed information downstream.
STAR Methods
CONTACT FOR REAGENT AND RESOURCE SHARING
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Marc H. Schieber (mschiebe@ur.rochester.edu).
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Non-human primates
Two male rhesus monkeys, L and X (weight 9 and 11 kg, age 11 and 13 years old, respectively), were subjects in the present study. All procedures for the care and use of these nonhuman primates followed the Guide for the Care and Use of Laboratory Animals and were approved by the University Committee on Animal Resources at the University of Rochester, Rochester, New York.
METHOD DETAILS
Behavioral task
Each monkey performed a reach, grasp, manipulate (RGM) task as described previously (Mollazadeh et al., 2011). In brief, four target objects (sphere, button, coaxial cylinder, perpendicular cylinder) were arranged at 45° intervals on a circle in front of the monkey (Figure 1A). Each target object was located at a radius of 13 cm from a central, home object (another coaxial cylinder). Figure 1C illustrates the sequence of temporal epochs in each trial. The monkey initiated a trial by pulling the home object for a variable initial hold period, during which blue LEDs around the pole supporting the home object were illuminated. These LEDs turned off at the end of the initial hold, and an instruction was delivered specifying which of the four objects the monkey must reach to, grasp, and manipulate to receive a water reward. A specific manipulation was required for each object: turn the sphere, push the button, pull the perpendicular cylinder, and pull the coaxial cylinder. Instructions were delivered either by illuminating the blue LEDs around the pole supporting the target object or by delivering a train of intracortical microstimulation (ICMS). During training sessions (Figure 2, from solid to dashed vertical lines), however, LED and ICMS instructions were delivered together. (Experimenters were not blind to the instruction condition.) After the onset of an instruction, the monkey was free to release the center object and reach to the target object. Upon contact, the monkey grasped and manipulated the target object, and held the manipulation for a fixed final hold period. ICMS instructions terminated upon contact with a target object (except when sweeping train duration), whereas LED instructions remained on until completion of the final hold. After successful completion of the final hold period, a water reward was delivered, and following an inter-trial interval (1000 ms) the monkey could initiate another trial by again pulling on the home object. Trials were presented in blocks of four, with each of the four objects instructed once per block and the order of the different objects re-randomized between each consecutive pair of blocks.
Errors occurred if the monkey did not hold the home object for the required initial hold duration (700 to 1,500 ms); failed to release the home object within an allowed reaction time (1,000 ms); contacted the wrong target object; failed to contact the correct target object within an allowed movement time (1,000 ms); or failed to hold the target object in the manipulated position for the entire final hold period (700 to 1,000 ms). These interval durations applied initially, when each monkey was using LED instructions alone. To minimize frustration while each monkey was learning to use ICMS instructions, the allowed reaction and movement times were increased. For monkey L, the allowed reaction and movement times were increased to 10,000 ms each, and then were reduced gradually to 4,000 ms each as performance improved. Note, however, that monkey L’s reaction time rarely exceeded 1,000 ms and movement time rarely exceeded 300 ms (Figure 2 and Table S2). For monkey X, the allowed reaction and movement times were increased to 5,000 and 7,000 ms, respectively, and then were reduced gradually to 3,000 and 3,000 ms, although reaction time rarely exceeded 3,000 ms and movement time, 500 ms. If an error occurred, the current trial aborted immediately, no reward was delivered, and the next trial instructed the same target object again. Error trials thus were repeated until performed successfully, and the monkey could have known which instruction would be delivered in any trial following an error trial. For analyses, we therefore used only trials for which the previous trial had been successful.
The RGM task was controlled by custom software written in TEMPO (Reflective Computing, Olympia, WA) which also generated behavioral event markers that were recorded in the data stream collected by a Plexon MAP Data Acquisition System (Plexon, Dallas, TX). These markers time-stamped behavioral events in the trial including: 1) initiation of the trial, 2) instruction onset, 3) movement onset (detected upon release of the home object), 4) target object contact (detected by strain gauges mounted on the horizontal rod supporting each object), 5) manipulation completion (detected by closure of a microswitch), 6) final hold completion, and 7) reward delivery.
Neural Electrodes and Intracortical Microstimulation
Both monkeys had been implanted with multiple floating microelectrode arrays (FMAs, Microprobes for Life Sciences, Gaithersburg, MD) in the premotor cortex (PM), primary somatosensory cortex (S1), and primary motor cortex (M1) of the left hemisphere (Figure 1D,E) as described previously (Mollazadeh et al., 2011). Each FMA had 16 electrodes (impedance ~0.5 MOhm, 70% Pt, 30% Ir) of various lengths to reach different depths down the banks of the arcuate or central sulcus (1.5 to 9 mm in PM, 1 to 6 mm in S1, 1.5 to 8 mm in M1). Because of the curvature of the buried cortex, however, we do not know the cortical layer in which each electrode tip came to lie. For the present study, we used 3 PM arrays in monkey L and 2 in monkey X, 4 S1 arrays in each monkey, and 1 M1 array in each monkey (Figure 1C,D).
To ensure that the electrode tips used for ICMS were in active cortex, in PM we selected electrodes that recorded single- or multi-unit activity while the monkey performed the RGM task using LED instructions. As illustrated in Figure 1F,G, PM neurons recorded through these electrodes typically were modulated during the RGM movements. In S1 we selected electrodes with neural responses to passive somatosensory stimulation. Four ICMS instructions were delivered initially through four arbitrarily-grouped sets of 3 to 4 electrodes in PM, or through four sets of 3 to 6 electrodes in S1 (Table S1) grouped on the basis of somatotopically similar somatosensory receptive fields (e.g. forearm versus fingers). No electrode was used for more than one instruction (i.e. in more than one set). As described in the Results, PM-ICMS instructions and M1-ICMS catch trial stimulation eventually were delivered through single electrodes. ICMS always consisted of symmetrical, biphasic, 200 μs/phase, cathodic-leading pulses delivered simultaneously through all electrodes of a given set at amplitudes ranging from 18 to 28 μA per electrode in PM or from 20 to 60 μA per electrode in S1 (Table S1).
ICMS was delivered using a constant current IZ2 Neural Stimulator controlled via an RZ5 BioAmp Processer hosted by a PC running the OpenEx Software Suite (Tucker-Davis Technologies, Gainesville, FL). The TEMPO software controlling the RGM task sent commands to the RZ5 that initiated the appropriate ICMS train at the end of the initial hold period in a given trial. Trains of ICMS pulses then were delivered by the IZ2 through the appropriate electrodes until another TEMPO command terminated the train. Except when sweeping train duration (see below), termination occurred when either: i) the correct target object was contacted, or ii) TEMPO software detected an error in the monkey’s behavioral performance.
Stimulus-triggered averaging (StimTA)
In certain sessions, dual cup electrodes with electrolyte gel were taped 1 to 2 cm apart on the skin overlying four muscle groups—triceps, biceps, forearm flexors, and forearm extensors—of the right arm, which the monkeys used in performing the RGM task. Bipolar signals from each electrode pair were amplified differentially with headstages (HST/8o50, 20× gain, 30 to 30,000 Hz bandpass; Plexon Inc.) and passed through a hardware preamplifier (PRA2/EMG-16-002, 50× gain, 300 to 3,000 Hz bandpass; Plexon). EMG signals were sampled at 5,000 Hz, amplified to a gain of 1,000× to 20,000× (PXI-6071E; National Instruments), and recorded as part of the datastream using Plexon’s Multichannel Acquisition Processor Software, which also recorded stimulus artifacts on FMA electrodes not used to deliver ICMS. EMGs were recorded in 2 (monkey L) or 4 (monkey X) sessions as the monkey performed using PM-ICMS instructions with intermixed M1-ICMS catch trials as described in the Results (see also Table S4).
In additional sessions performed several months after completion of all other studies, we conducted an expanded search to determine whether ICMS delivered in PM might have evoked responses at currents higher than we had used and/or in muscles we had not monitored while delivering PM-ICMS instructions. Focusing on those electrodes used when delivering PM-ICMS through single electrodes, we delivered jittered ICMS pulses at the current used for PM-ICMS instructions in each monkey (20 μA for monkey L, 25 μA for monkey X), at 40 μA, and at 55 μA in separate blocks of trials and recorded EMG activity from four additional muscles that sampled the shoulder and head—infraspinatus, anterior deltoid, post-auricular, and frontalis. Rather than re-training the monkeys to use PM-ICMS instructions at each current level, both monkeys used LED instructions to perform the RGM task while jittered ICMS pulses were delivered through each of the 4 PM electrodes and 1 of the M1 electrodes used previously for catch trials.
Conventional, short-train ICMS
In an additional session, we tranquilized each monkey with ketamine (5–10 mg/kg IM) and delivered trains of 12 biphasic, cathodal-first pulses, 200 μs per phase, at 333 Hz and currents up to 100 μA, using a biphasic pulse generator and stimulus isolator (BPG-1 and BSI-1, BAK Electronics, Inc., Umatilla, FL). Currents were monitored by measuring the voltage drop across a 100 Ω in-series resistor with a high-impedance amplifier (DAM80, World Precision Instruments, Sarasota, FL). As trains were generated by a micro1401 interface (Cambridge Electronic Design, Cambridge, UK) at random intervals between 1 and 4 seconds, we observed and palpated the animal’s face, upper extremity, trunk, and lower extremity for evoked twitches.
QUANTIFICATION AND STATISTICAL ANALYSIS
Performance Metrics
Offline, using MATLAB (MathWorks, Natick, MA), we examined three different performance metrics. First, for each session we determined the success rate by counting the number of successful trials preceded by a successful trial, Nsuccess, and the number of error trials preceded by a successful trial, Nerror. Success rate then was calculated as a percentage: 100×(Nsuccess/(Nsuccess + Nerror)). Second, reaction times were measured in these correctly performed trials (Nsuccess) as the duration from instruction onset to movement onset. Third, movement times were measured in the same correctly performed trials (Nsuccess) as the duration from movement onset to target object contact.
PM-ICMS Parameter Sweeps
Once each monkey had learned to perform the RGM task with PM-ICMS instructions, three stimulation parameters—periodic pulse frequency, current amplitude, and train duration—were swept to determine the values of each parameter at which performance deteriorated (Figure 3, Table S3). When not being swept, i) frequency was not periodic but instead the inter-pulse intervals were jittered stochastically from 12.5 to 40 ms for monkey L or from 12.5 to 35 ms for monkey X, ii) amplitudes were fixed at values ranging from 10 to 28 μA per electrode, and iii) pulse trains continued until a target object was contacted. Stimulation frequencies were swept from 20 to 100 Hz; amplitudes were swept from 2 to 20 μA for monkey L and from 5 to 25 μA for monkey X; and train durations were swept from 50 to 1000 ms or object contact, whichever came first. Success rate, reaction time, and movement time were examined for each stimulation parameter sweep. Success rates were fit to a logistic function:
where f (x) represents the success rate at parameter value x; a, the steepness of the curve; and b, the point at which success rates crossed 50%. A binomial distribution was fit at each swept value to estimate the median success rate along with the 25th and 75th percentiles.
Post-stimulus effects (PStEs)
Off-line, the times of ICMS pulses from the stimulus-triggered averaging sessions were identified by sorting the stimulation artifacts on an FMA recording channel that provided clean isolation using Plexon’s Offline Sorter. This discrimination process resulted in trigger times being defined up to 2 ms after the onset of the stimulation artifacts. Because ICMS pulses were delivered at intervals of 12.5 to 30 or 40 ms, the trigger pulses were followed frequently by another artifact during the interval when PStEs could occur. To eliminate distortion of the average by these artifacts, we blanked each original EMG signal from 2 ms before to 4 ms after each trigger time by replacing the EMG data values during each such interval with MATLAB “NaNs” (Not-a-Number). We then formed StimTAs of each EMG recording by rectifying the data values, aligning the data from 30 ms before to 50 ms after each trigger time, and then averaging the data values in each aligned bin, using MATLAB’s “nanmean” function to account for the number of blanked data values. StimTAs then were smoothed with a flat five-point finite impulse response filter. Any underlying baseline trend was estimated with a bootstrap approach (Perel et al., 2014). More specifically, each StimTA was recomputed 100 times using trigger times that had been jittered with Gaussian noise (σ = 10 ms), providing an estimate of the baseline trend as a pointwise mean and standard deviation. The mean trend was subtracted from the original StimTA, and this mean-adjusted StimTA was z-scored, dividing by the point-wise standard deviation. A muscle was considered to have a significant PStE if, following the stimulation artifact (i.e. > 4.0 ms after the aligned trigger times), the adjusted StimTA was more than 3 standard deviations from the baseline trend for at least 1 ms (5 consecutive bins) (Park et al., 2004).
Sample Size
We did not perform a priori power analyses to determine sample size because little prior information was available on which to base an estimate of learning and performance in the novel situation of PM-ICMS instructions. Rather, our dataset incorporates multiple sessions from each of 2 monkeys: monkey L, 92 S1-ICMS sessions, 85 PM-ICMS; monkey X, 143 PM-ICMS sessions, 24 S1-ICMS. Individual sessions included a median of 988 [911, 1078] (25th and 75th percentiles) individual RGM trials.
Statistical Tests
Success rates in trials instructed with PM-ICMS, S1-ICMS, or LEDs were compared using X2 tests with two-sided Bonferroni-corrected post-hoc X2 tests. Both reaction times and movement times were compared using non-parametric Kruskal-Wallis tests with two-sided Tukey’s honest significant difference post-hoc tests. Success rates, reaction times, and movement times, all are reported as medians with 25th and 75th percentiles.
DATA AND SOFTWARE AVAILABILITY
Analysis-specific code and data are available by request to the Lead Contact: Marc H. Schieber, mschiebe@ur.rochester.edu.
Supplementary Material
Acknowledgments
This work was supported by grants F32NS093709 to KAM and R01NS092626 to MHS from the NINDS. The authors declare no competing financial interests. The authors thank Marsha Hayles for editorial comments.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions: Conceptualization, Methodology, Investigation, Writing–(Original Draft, Review, Editing), Funding Acquisition, KAM and MHS; Resources and Supervision, MHS
References
- Andersen P, Hagan PJ, Phillips CG, Powell TP. Mapping by microstimulation of overlapping projections from area 4 to motor units of the baboon’s hand. Proceedings of the Royal Society of London - Series B: Biological Sciences. 1975;188:31–36. doi: 10.1098/rspb.1975.0002. [DOI] [PubMed] [Google Scholar]
- Baldwin MK, Cooke DF, Krubitzer L. Intracortical Microstimulation Maps of Motor, Somatosensory, and Posterior Parietal Cortex in Tree Shrews (Tupaia belangeri) Reveal Complex Movement Representations. Cerebral cortex. 2017;27:1439–1456. doi: 10.1093/cercor/bhv329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouton CE, Shaikhouni A, Annetta NV, Bockbrader MA, Friedenberg DA, Nielson DM, Sharma G, Sederberg PB, Glenn BC, Mysiw WJ, et al. Restoring cortical control of functional movement in a human with quadriplegia. Nature. 2016;533:247–250. doi: 10.1038/nature17435. [DOI] [PubMed] [Google Scholar]
- Capogrosso M, Milekovic T, Borton D, Wagner F, Moraud EM, Mignardot JB, Buse N, Gandar J, Barraud Q, Xing D, et al. A brain-spine interface alleviating gait deficits after spinal cord injury in primates. Nature. 2016;539:284–288. doi: 10.1038/nature20118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dadarlat MC, O’Doherty JE, Sabes PN. A learning-based approach to artificial sensory feedback leads to optimal integration. Nature neuroscience. 2015;18:138–144. doi: 10.1038/nn.3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmurget M, Reilly KT, Richard N, Szathmari A, Mottolese C, Sirigu A. Movement intention after parietal cortex stimulation in humans. Science. 2009;324:811–813. doi: 10.1126/science.1169896. [DOI] [PubMed] [Google Scholar]
- Desmurget M, Richard N, Harquel S, Baraduc P, Szathmari A, Mottolese C, Sirigu A. Neural representations of ethologically relevant hand/mouth synergies in the human precentral gyrus. Proc Natl Acad Sci USA. 2014;111:5718–5722. doi: 10.1073/pnas.1321909111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ethier C, Oby ER, Bauman MJ, Miller LE. Restoration of grasp following paralysis through brain-controlled stimulation of muscles. Nature. 2012;485:368–371. doi: 10.1038/nature10987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzsimmons NA, Drake W, Hanson TL, Lebedev MA, Nicolelis MA. Primate reaching cued by multichannel spatiotemporal cortical microstimulation. The Journal of neuroscience. 2007;27:5593–5602. doi: 10.1523/JNEUROSCI.5297-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flesher SN, Collinger JL, Foldes ST, Weiss JM, Downey JE, Tyler-Kabara EC, Bensmaia SJ, Schwartz AB, Boninger ML, Gaunt RA. Intracortical microstimulation of human somatosensory cortex. Sci Transl Med. 2016;8:361ra141. doi: 10.1126/scitranslmed.aaf8083. [DOI] [PubMed] [Google Scholar]
- Fried I, Katz A, McCarthy G, Sass KJ, Williamson P, Spencer SS, Spencer DD. Functional organization of human supplementary motor cortex studied by electrical stimulation. The Journal of neuroscience. 1991;11:3656–3666. doi: 10.1523/JNEUROSCI.11-11-03656.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu QJ, Galvin JJ., 3rd Perceptual learning and auditory training in cochlear implant recipients. Trends Amplif. 2007;11:193–205. doi: 10.1177/1084713807301379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentilucci M, Fogassi L, Luppino G, Matelli M, Camarda R, Rizzolatti G. Functional organization of inferior area 6 in the macaque monkey. I. Somatotopy and the control of proximal movements. ExpBrain Res. 1988;71:475–490. doi: 10.1007/BF00248741. [DOI] [PubMed] [Google Scholar]
- Germain L, Lamarre Y. Neuronal activity in the motor and premotor cortices before and after learning the associations between auditory stimuli and motor responses. Brain Res. 1993;611:175–179. doi: 10.1016/0006-8993(93)91792-q. [DOI] [PubMed] [Google Scholar]
- Grafton ST. The cognitive neuroscience of prehension: recent developments. Exp Brain Res. 2010;204:475–491. doi: 10.1007/s00221-010-2315-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graziano MS. Where is my arm? The relative role of vision and proprioception in the neuronal representation of limb position. Proc Natl Acad Sci U S A. 1999;96:10418–10421. doi: 10.1073/pnas.96.18.10418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graziano MS. Ethological Action Maps: A Paradigm Shift for the Motor Cortex. Trends Cogn Sci. 2016;20:121–132. doi: 10.1016/j.tics.2015.10.008. [DOI] [PubMed] [Google Scholar]
- Graziano MS, Taylor CS, Moore T. Complex movements evoked by microstimulation of precentral cortex. Neuron. 2002;34:841–851. doi: 10.1016/s0896-6273(02)00698-0. S0896627302006980 [pii] [DOI] [PubMed] [Google Scholar]
- Griffin DM, Hudson HM, Belhaj-Saif A, Cheney PD. EMG activation patterns associated with high frequency, long-duration intracortical microstimulation of primary motor cortex. J Neurosci. 2014;34:1647–1656. doi: 10.1523/JNEUROSCI.3643-13.2014. 10.1523/JNEUROSCI.3643-13.201434/5/1647[pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karl JM, Whishaw IQ. Different evolutionary origins for the reach and the grasp: an explanation for dual visuomotor channels in primate parietofrontal cortex. Front Neurol. 2013;4:208. doi: 10.3389/fneur.2013.00208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Callier T, Tabot GA, Gaunt RA, Tenore FV, Bensmaia SJ. Behavioral assessment of sensitivity to intracortical microstimulation of primate somatosensory cortex. Proc Natl Acad Sci USA. 2015a;112:15202–15207. doi: 10.1073/pnas.1509265112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Callier T, Tabot GA, Tenore FV, Bensmaia SJ. Sensitivity to microstimulation of somatosensory cortex distributed over multiple electrodes. Frontiers in systems neuroscience. 2015b;9:47. doi: 10.3389/fnsys.2015.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan HC, MacKay WA, Murphy JT, Wong YC. Spatial organization of precentral cortex in awake primates. II. Motor outputs. J Neurophysiol. 1978;41:1120–1131. doi: 10.1152/jn.1978.41.5.1120. [DOI] [PubMed] [Google Scholar]
- Lewis PM, Ackland HM, Lowery AJ, Rosenfeld JV. Restoration of vision in blind individuals using bionic devices: a review with a focus on cortical visual prostheses. Brain Res. 2015;1595:51–73. doi: 10.1016/j.brainres.2014.11.020. [DOI] [PubMed] [Google Scholar]
- Mitz AR, Godschalk M, Wise SP. Learning-dependent neuronal activity in the premotor cortex: activity during the acquisition of conditional motor associations. Journal of Neuroscience. 1991;11:1855–1872. doi: 10.1523/JNEUROSCI.11-06-01855.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollazadeh M, Aggarwal V, Davidson AG, Law AJ, Thakor NV, Schieber MH. Spatiotemporal variation of multiple neurophysiological signals in the primary motor cortex during dexterous reach-to-grasp movements. The Journal of neuroscience. 2011;31:15531–15543. doi: 10.1523/JNEUROSCI.2999-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphey DK, Maunsell JH. Electrical microstimulation thresholds for behavioral detection and saccades in monkey frontal eye fields. Proceedings of the National Academy of Sciences. 2008;105:7315–7320. doi: 10.1073/pnas.0710820105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mushiake H, Inase M, Tanji J. Neuronal activity in the primate premotor, supplementary, and precentral motor cortex during visually guided and internally determined sequential movements. J Neurophysiol. 1991;66:705–718. doi: 10.1152/jn.1991.66.3.705. [DOI] [PubMed] [Google Scholar]
- O’Doherty JE, Lebedev MA, Hanson TL, Fitzsimmons NA, Nicolelis MA. A brain-machine interface instructed by direct intracortical microstimulation. Frontiers in integrative neuroscience. 2009;3:20. doi: 10.3389/neuro.07.020.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohbayashi M, Picard N, Strick PL. Inactivation of the Dorsal Premotor Area Disrupts Internally Generated, But Not Visually Guided, Sequential Movements. J Neurosci. 2016;36:1971–1976. doi: 10.1523/JNEUROSCI.2356-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overduin SA, d’Avella A, Carmena JM, Bizzi E. Microstimulation activates a handful of muscle synergies. Neuron. 2012;76:1071–1077. doi: 10.1016/j.neuron.2012.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park MC, Belhaj-Saif A, Cheney PD. Properties of primary motor cortex output to forelimb muscles in rhesus macaques. Journal of Neurophysiology. 2004;92:2968–2984. doi: 10.1152/jn.00649.2003. [DOI] [PubMed] [Google Scholar]
- Penfield W, Rasmussen T. The Cerebral Cortex of Man (MacMillan) 1950 [Google Scholar]
- Perel S, Schwartz AB, Ventura V. Single-snippet analysis for detection of postspike effects. Neural computation. 2014;26:40–56. doi: 10.1162/NECO_a_00531. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Camarda R, Fogassi L, Gentilucci M, Luppino G, Matelli M. Functional organization of inferior area 6 in the macaque monkey. II. Area F5 and the control of distal movements. ExpBrain Res. 1988;71:491–507. doi: 10.1007/BF00248742. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Scandolara C, Matelli M, Gentilucci M. Afferent properties of periarcuate neurons in macaque monkeys. I. Somatosensory responses. Behav Brain Res. 1981a;2:125–146. doi: 10.1016/0166-4328(81)90052-8. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Scandolara C, Matelli M, Gentilucci M. Afferent properties of periarcuate neurons in macaque monkeys. II. Visual responses. Behav Brain Res. 1981b;2:147–163. doi: 10.1016/0166-4328(81)90053-x. [DOI] [PubMed] [Google Scholar]
- Romo R, Hernandez A, Zainos A. Neuronal correlates of a perceptual decision in ventral premotor cortex. Neuron. 2004;41:165–173. doi: 10.1016/s0896-6273(03)00817-1. [DOI] [PubMed] [Google Scholar]
- Romo R, Hernandez A, Zainos A, Salinas E. Somatosensory discrimination based on cortical microstimulation. Nature. 1998;392:387–390. doi: 10.1038/32891. [DOI] [PubMed] [Google Scholar]
- Stepniewska I, Fang PC, Kaas JH. Microstimulation reveals specialized subregions for different complex movements in posterior parietal cortex of prosimian galagos. Proc Natl Acad Sci USA. 2005;102:4878–4883. doi: 10.1073/pnas.0501048102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabot GA, Dammann JF, Berg JA, Tenore FV, Boback JL, Vogelstein RJ, Bensmaia SJ. Restoring the sense of touch with a prosthetic hand through a brain interface. Proc Natl Acad Sci USA. 2013;110:18279–18284. doi: 10.1073/pnas.1221113110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troyk P, Bak M, Berg J, Bradley D, Cogan S, Erickson R, Kufta C, McCreery D, Schmidt E, Towle V. A model for intracortical visual prosthesis research. Artificial organs. 2003;27:1005–1015. doi: 10.1046/j.1525-1594.2003.07308.x. [DOI] [PubMed] [Google Scholar]
- Weinrich M, Wise SP. The premotor cortex of the monkey. Journal of Neuroscience. 1982;2:1329–1345. doi: 10.1523/JNEUROSCI.02-09-01329.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


