Abstract
Objective
Diets high in saturated fat induce obesity and insulin resistance, and impair insulin access to skeletal muscle, leading to reduced insulin levels at the muscle cell surface available to bind insulin receptors and induce glucose uptake. In contrast, diets supplemented with polyunsaturated fat improve insulin sensitivity and reduce the risk for type 2 diabetes. We hypothesized that a polyunsaturated high fat diet would preserve insulin sensitivity and insulin access to muscle as compared to a saturated high fat diet.
Methods
After 12 weeks of control, saturated (LARD) or polyunsaturated (salmon oil; SO) high fat diet, we measured muscle insulin sensitivity and insulin access to skeletal muscle using lymph, a surrogate of skeletal muscle interstitial fluid.
Results
Fat diets induced similar weight gain, yet only LARD impaired insulin sensitivity. Hyperinsulinemia in the LARD group did not induce an increase in basal interstitial insulin, suggesting reduced insulin access to muscle after LARD, but not SO.
Conclusions
A diet high in polyunsaturated fat does not impair insulin access to muscle interstitium or induce insulin resistance as observed with a saturated fat diet, despite similar weight gain. Future studies should determine whether dietary SO supplementation improves impairments in insulin access to skeletal muscle.
Introduction
Endothelial dysfunction is suggested to be an early complication of obesity (1, 2, 3) and is associated with the metabolic syndrome (4), pre-diabetes (5), and is present in people with a family history of diabetes (6). The endothelium is involved in insulin action, since insulin must cross the capillary endothelium to access the interstitial space where it can bind receptors to initiate insulin signaling (7, 8). Therefore, factors that affect endothelial function and thus insulin delivery to the surface of the cell may directly impact insulin sensitivity. In the case of women with obesity, higher circulating levels of insulin have been reported as compared to lean women, yet no differences exist in interstitial insulin levels in adipose tissue and skeletal muscle, suggesting impaired insulin access to the interstitial space (9). In addition, men with obesity have slower transcapillary transport of insulin as compared to lean men (10), further suggesting a reduced ability of insulin to cross the interstitial space to act on peripheral tissues during obesity. Our results in the canine model support these human findings and demonstrate that impaired insulin access under basal insulin levels develops with long-term fat feeding (11), but that this effect is overcome at higher insulin levels.
Dietary fat can induce endothelial dysfunction (12). In contrast, polyunsaturated fats, including omega-3s, have been shown to have beneficial effects on endothelial function in a variety of different models (13, 14). To test the hypothesis that insulin access to skeletal muscle is preserved in a model of obesity induced by a diet high in polyunsaturated fat, we performed hyperinsulinemic euglycemic clamps to assess insulin sensitivity and measured interstitial insulin in dogs fed a normal diet, a diet high in saturated fat or a diet high in polyunsaturated fat. We observed marked differences among these diets in their effects on insulin action as well as insulin movement across the capillary endothelial barrier.
Methods
Animals
Male mongrel dogs (Antech, Barnhart, MO, USA, >1 year old) were housed in the Cedars-Sinai Medical Center vivarium under controlled kennel conditions (12h:12h light:dark cycle). Animals were accepted into the study following physical examination and a comprehensive panel of blood tests and were included in the study only if judged to be in good health as determined by visual observation, body temperature and hematocrit. Protocols were conducted in conformity with the Public Health Service (PHS) Policy on Humane Care and Use of Laboratory Animals, and were approved by the Cedars-Sinai Medical Center Institutional Animal Care and Use Committee.
Diet
The control group (CON, n=8) was fed a standard ad libitum diet, consisting of dry chow (40% carbohydrate, 26% protein, 14% fat and 3% fiber [mixture of Laboratory HDL Canine Diet and Prolab Canine 2000, Richmond IN, USA]).
For the high-fat diet groups, food was presented from 9:00am to 10:00am each day. Animals were fed a daily diet of one can of Hill’s Prescription Diet (415g; 10% carbohydrate, 9% protein 8% fat, 0.3% fiber and 73% moisture [Hill’s Pet Nutrition, Topeka KS, USA]) and 825g dry chow (40% carbohydrate, 26% protein, 14% fat and 3% fiber [mixture of Laboratory HDL Canine Diet and Prolab Canine 2000, Richmond IN, USA]), supplemented with either 6g/kg of rendered pork fat (LARD, n=8) or salmon oil (SO, n=8). These alternative diets contained identical macronutrient content consisting of 21,025kJ/day comprised of 27% carbohydrate, 19% protein and 53% fat. The added lard consisted of 38.5% saturated fatty acid, 10.8% polyunsaturated fatty acid (10% ω-6; 0.8% ω-3), 44.6% monounsaturated fatty acid, and 0.1% cholesterol. The Salmon oil addition consists of 19.9% saturated fatty acid, 40.4% polyunsaturated fatty acid (1.5% ω-6; 35.5% ω-3).
The acute effects of six weeks of LARD and SO diets on insulin sensitivity and cardiac function have been reported previously (15). The present study reports data on extended diet durations of 3.0±0.3 months of lard feeding and 3.9±0.1 months of salmon oil feeding.
Hyperinsulinemic euglycemic clamp
Clamps were done under anesthesia; animals were fasted overnight (for ad lib fed animals, food was removed at noon), then at 8 am were sedated with acepromazine maleate (Prom-Ace, Aueco, Fort Dodge, IA; 0.22mg/kg) and atropine sulfate (Western Medical, Arcadia, CA; 0.11 mL/kg). Anesthesia was induced with sodium pentobarbital (Western Medical, Arcadia, CA; 0.5mL/kg) or propofol (Western Medical, Arcadia, CA; 6mg/kg) and maintained with inhaled isofluorane or sevoflurane (Western Medical, Arcadia, CA). Dogs were placed on heating pads to maintain body temperature. Intracatheters were inserted into the left cephalic vein for variable glucose infusion and the right cephalic vein for insulin, somatostatin, and tracer infusion. Indwelling catheters were placed into the left femoral artery and vein for sampling. The hindlimb lymphatic vessel was cannulated by placing a polyethylene catheter (PE10) into the afferent lymphatic vessel of the deep inguinal lymph node. Lymph was collected by gently massaging the leg directly above the popliteal area, which has been shown to instantaneously increase lymph drainage without affecting lymph or plasma oncotic pressures (16). Blood pressure (cuff on opposite hind leg), heart rate, O2 saturation and CO2 were monitored continuously. The surgery was completed at approximately 10am, at which time the experiment began.
Immediately after the completion of the surgical procedures and sampling of fasting lymph and plasma, somatostatin was infused to inhibit endogenous insulin secretion (1ug/min/kg; Bachem) (at time = −180 min), and basal insulin was replaced systemically (0.2mU/min/kg; Novo Nordisk, Bagsvaerd, Denmark) and continuously for the remainder of the study. Exogenous 20% glucose was infused into the left cephalic vein at variable rates to clamp arterial glucose to basal levels throughout the experimental period. Plasma samples were taken from the femoral artery and femoral vein every 10–15 minutes. Lymph vessels were sampled by gently massaging the hindlimb distal to the site of catheterization. After 180 minutes of insulin replacement (time = 0 min), the insulin concentration was increased to 1.2mU/min/kg, which was continued for the remainder of the study. Insulin levels from the artery, vein and lymph were averaged over the last 30 minutes of the clamp to examine differences in insulin access at steady state. At the conclusion of these experiments, animals were euthanized with an overdose of sodium pentobarbital (Eutha-6, Western Medical; 65mg/kg).
Assays
Arterial, venous and lymph samples were collected in microtubes pre-coated with lithium-heparin (Becton Dickinson, Franklin Lakes, NJ). Arterial and venous tubes also contained 50μL EDTA (Sigma Chemicals, St Louis, MO). Blood samples were centrifuged immediately and the supernatant was transferred. Plasma and lymph samples were immediately assayed for glucose with a YSI 2700 autoanalyzer (Yellow Springs Instrument Co., Yellow Springs, OH) before freezing at −20°C until further analysis. Insulin was measured in plasma and lymph with an ELISA developed for dog plasma (Alpco, Salem, NH).
Tissue insulin sensitivity
Tissue insulin sensitivity (Stissue) reflects the insulin-mediated glucose uptake in response to the local, rather than systemic, insulin concentration and thus indicates the ability of the muscle to respond to interstitial insulin. Stissue was calculated from the arterio-venous glucose difference (ΔAVGlu), the change in interstitial insulin (ΔInsI), and the glucose concentration (GlucSS) at steady state: ΔAVGlu/(ΔInsI x GlucSS).
Statistical analyses
Experimental data are shown as mean ± SEM. Statistical analyses were performed with paired or unpaired Student’s t tests, or one-way or two-way ANOVAs with Tukey’s pairwise comparisons, as appropriate (GraphPad Prism version 5.04 for Windows, GraphPad Software, San Diego, CA). An interquartile test for outliers was used. All differences were considered statistically significant when p<0.05.
Results
Baseline characteristics of diets
Dietary fat supplementation for 3 months induced an increase in weight of 6.4 ±3.4% and 8.3±1.5% in lard and salmon oil fed dogs, respectively, which was not different between fat fed groups (P=0.7). Glucose levels assessed in arterial plasma under anesthesia prior to the clamp were not different between groups (CON 99.7±3.4, LARD 97.3±2.0, SO 103.6±4.1 mg/dl), and similarly, no changes in lymph glucose levels were noted (CON 109.6±4.9, LARD 103.4±2.0, SO 110.9±6.0 mg/dl).
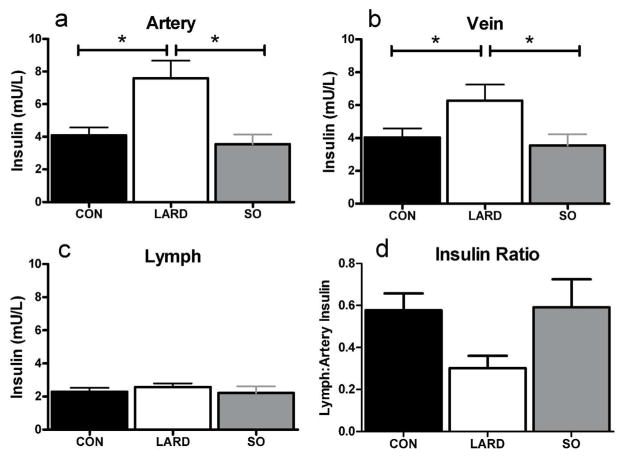
Previously published findings after six weeks in each diet condition reported that plasma insulin was significantly increased from baseline from 50.4±7.7 to 60.4±10.7 pmol/l in LARD animals, while the SO intervention caused no significant changes in insulin (34.6±6.8 to 31.0±4.3 pmol/l) (15). Similarly, in these longer studies we found that lard, but not salmon oil feeding induced plasma hyperinsulinemia at basal insulin levels in both artery and vein (Figure 1a,b). However, elevated plasma insulin levels in the LARD group did not translate to an elevation in interstitial insulin levels in contrast to chow-fed lean animals (Figure 1c), indicating reduced insulin access to the interstitium.
Figure 1.
Insulin levels in artery (a), vein (b) and lymph (c), and ratio of lymph:artery insulin under fasting conditions in CON, LARD and SO. Statistics performed with one way ANOVA, * represents significance at p<0.05.
Insulin sensitivity measures
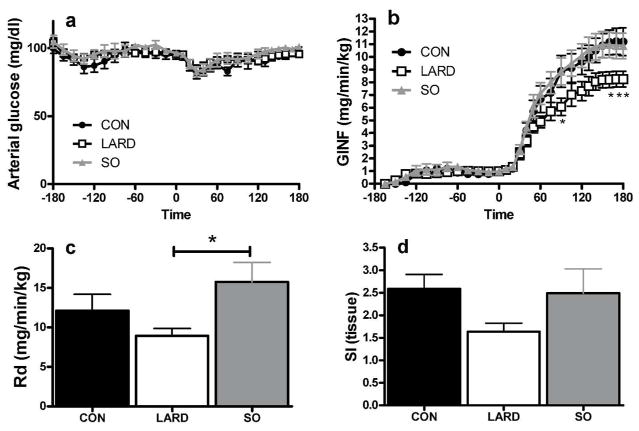
To ensure that animals were not differentially stressed at the start of the experiment as compared to the end we confirmed no significant different between heart rate, blood pressure or blood flow at baseline compared to steady state (Table 1), and oxygen saturation was maintained at 98–99% in all groups. Plasma glucose concentrations during the clamp were maintained at euglycemia and were not different between groups (Figure 2a). However, to maintain euglycemia, a lower glucose infusion rate was required for LARD animals compared to CON (Figure 2b). In contrast, glucose infusion was not different in SO animals as compared to CON. Peripheral glucose disappearance rate (Rd) (Figure 2c) was also reduced in the LARD group, indicating impaired insulin sensitivity.
Table 1.
Effects of diet on heart rate, BP, femoral blood flow, insulin sensitivity, insulin clearance, and pre-clamp free fatty acid levels. Means ± SEM, statistics performed with one way ANOVA, significance assumed at p<0.05.
| CON | LARD | SO | |
|---|---|---|---|
| Baseline Heart Rate | 95.8±7.4 | 108.9±5.5 | 95.6±4.3 |
| Baseline Blood Pressure | 100/50 (70+4) | 108/60 (72±5) | 106/54 (75±2) |
| Baseline Femoral Blood flow | 155.6±29.8 | 132.3±26.0 | 147.3±18.0 |
| Clamp Heart Rate | 107.5±6.5 | 103.8±5.0 | 107.5±3.8 |
| Clamp Blood Pressure | 95/47 (67±2) | 102/48 (64±4) | 102/51 (72±1) |
| Clamp Femoral Blood Flow | 202.1±15.8 | 179.6±23.2 | 174.0±21.2 |
|
| |||
| SIGinf (dl/min/kg per LU/ml x 104) | 18.2±2.8 | 10.5±1.5 * | 14.6±1.8 |
| SIRd (dl/min/kg per LU/ml x 104) | 17.4±3.6 | 8.1±5.4 | 17.4±4.0 |
| SIEGP (dl/min/kg per LU/ml x 104) | −0.4±3.0 | −0.4±1.1 | 1.1±1.4 |
| MCR (ml/min/kg) | 16.9±1.6 | 13.8±0.8 | 15.0±1.0 |
| FFA (arterial) (mmol/L) | 0.14±0.04 | 0.14±0.02 | 0.14±0.04 |
| FFA (lymph) (mmol/L) | 0.62±0.28 | 0.30±0.03 | 0.21±0.06 |
Figure 2.
Glucose levels in artery (a) were maintained by infusing exogenous glucose at variable rates (GINF) (b). Insulin infusion began at time 0, and induced changes in glucose disappearance (Rd)(c), and tissue insulin sensitivity (d) in CON, LARD and SO. One-way or two-way RM ANOVA as appropriate, *p<0.05 compared to SO.
Tissue insulin sensitivity, calculated from the arteriovenous glucose removal across the leg and the interstitial insulin concentration, showed a trend to be impaired in LARD animals only (p=0.09) (Figure 2d).
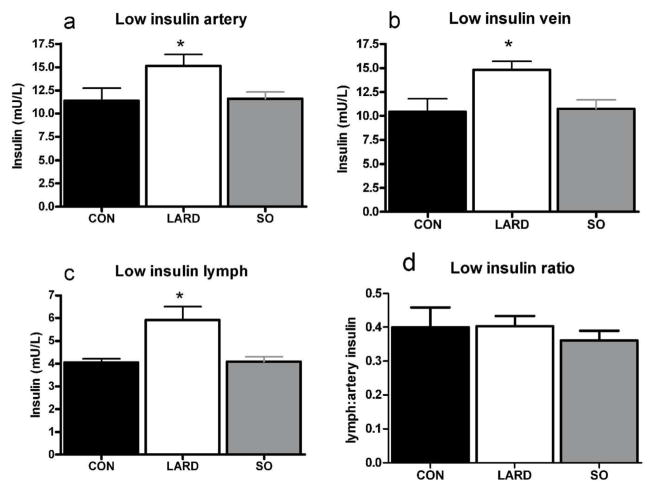
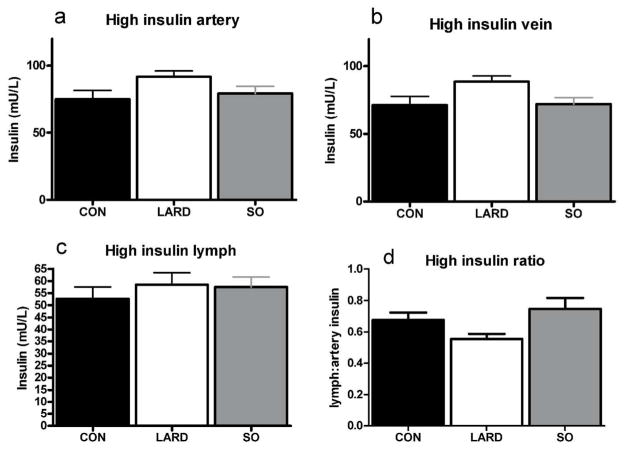
In contrast to fasting plasma hyperinsulinemia in conjunction with unchanged interstitial insulin levels, exogenous insulin infusion at both stages of the clamp elevated interstitial insulin levels (Figure 3 and Figure 4). Plasma insulin levels during the clamp were significantly higher in LARD animals at low insulin levels, (Figure 3) and showed a similar trend at high insulin levels (Figure 4). However, the same insulin doses (0.2 and 1.2mU/min/kg) were administered to all animals, suggesting a trend for a decrease in the metabolic clearance rate of insulin in the LARD group (MCR, Table 1).
Figure 3.
Insulin levels at steady state in artery (a,), vein (b) and lymph (c) after infusion of low insulin (0.2mU/min/kg insulin) in CON, LARD and SO. Insulin ratio of lymph:artery is also shown (d) Significance is calculated by one way ANOVA, significance is assumed when p<0.05, *p<0.05 vs CON.
Figure 4.
Insulin levels at steady state in artery (a), vein (b) and lymph (c) after infusion of high insulin (1.2mU/min/kg insulin) in CON, LARD and SO. Insulin ratio of lymph:artery is also shown (d).
LARD animals displayed a significantly reduced insulin sensitivity compared to CON animals, as measured by glucose infusion (SIginf) during the clamp, whereas there was no difference in SIginf in SO animals compared to CON (Table 1). A similar, but non-significant trend was seen for reduced insulin sensitivity as measured by the rate of glucose disappearance (SIRd). There was no effect of diet on endogenous glucose production by the liver (SIEGP), consistent with previous findings in anesthetized animals. We also observed no significant effects of any diet on plasma or lymph FFA levels.
Discussion
Diets rich in polyunsaturated fatty acid (PUFA) have been proposed to have beneficial effects on the endothelium. Here we report that a PUFA diet, while inducing a significant weight gain, did not induce insulin resistance, or impair insulin movement into the interstitial space under basal insulin levels, as compared with the LARD diet. Our previously published results also demonstrate that a LARD diet induces insulin resistance and impairs insulin access to the skeletal muscle interstitium under basal, non-insulin stimulated conditions (11), but not during higher levels of exogenously infused insulin. Thus, the present results support the concept that polyunsaturated fatty acid diets do not have the detrimental effects on insulin action seen with saturated fatty acid diets, even in the context of comparable obesity.
We have extensive experience using a well-established canine model to demonstrate that diets supplemented with lard induce insulin resistance (15, 17, 18, 19) within a relatively short time frame (20). We have also shown that a diet supplemented with salmon oil induces similar weight gain to a lard diet, but is not associated with impairments in insulin sensitivity (15). In mouse studies, a diet high in saturated fat and subsequently enriched with omega-3 PUFAs reversed glucose intolerance and vascular dysfunction and improves insulin signaling (21, 22).
Omega-3 supplementation may improve vascular function in part through changes in fatty acid composition (14) and activation of AMP-activated protein kinase (23). Conversely, hyperlipidemia is normally thought to impair vascular function (1, 24). As we and others have shown, insulin must cross the endothelial barrier to bind to insulin receptors on the muscle cells to initiate insulin-mediated glucose uptake. However, prior to crossing the endothelium, capillary recruitment may also occur to more fully perfuse the muscle with blood, and more efficiently deliver insulin and glucose to the muscle cell. Blocking capillary recruitment can impair insulin and glucose delivery as well. FFA levels affect capillary recruitment, which may contribute to some of the insulin resistance induced by elevated circulating FFA (21). However, we did not see changes in FFA levels in our study, indicating that elevated plasma FFA does not directly impair insulin access in this model. The lard based diet has been extensively studied in our laboratory, and we have shown that while there are no changes in fasting or fed FFA levels, we do detect a nocturnal increase in plasma FFA content (25). While it is possible that elevated nocturnal FFA may induce endothelial dysfunction and therefore impair capillary recruitment, we did not measure nocturnal FFA in this study. Further studies are needed to determine whether saturated fat indeed restricts capillary function as it relates to insulin access in this model.
One mechanism by which omega-3s may preserve glucose tolerance has been suggested to be by preventing accumulation of lipid intermediates that interfere with mitochondrial function (26). In skeletal muscle, mitochondria can adapt to a saturated fat diet by reducing proton leak, whereas proton leak is increased with diets high in omega-3 PUFAs (27), resulting in differential effects of these dietary fats on energy conservation and expenditure. In addition, there are distinct effects of dietary fat on mitochondrial enzyme activity, fission proteins and apoptotic signaling (27), however, we did not investigate these mechanisms of insulin resistance in the current study.
Hyperinsulinemic compensation has been hypothesized to be a normal response to insulin resistance (28). Hyperinsulinemia can be driven by changes in insulin secretion and/or insulin clearance (20). Indeed, insulin resistance develops in LARD animals and therefore hyperinsulinemic compensation achieved by increased insulin secretion (15) and/or decreased metabolic clearance rate of insulin, is necessary to produce adequate glucose disposal. Reduced insulin clearance in LARD animals is supported by our finding that the same amount of insulin infused into each group of animals led to higher plasma insulin concentrations in LARD animals only. Salmon oil feeding did not induce insulin resistance at the whole body (SIGINF), peripheral (SIRd) or tissue level (SItissue), and as such, hyperinsulinemic compensation was not required. Lymph is generally accepted to be an appropriate measure of interstitial fluid, and results are similar to other methods of interstitial sampling (29). The lymph vessel is in fact very similar in structure to blood vessels, thus things that induce endothelial dysfunction may also have effects on lymph function. In fact, recent studies have implicated lymphatic dysfunction in metabolic syndrome (30, 31, 32) in addition to endothelial dysfunction. In future studies we plan to further confirm the use of lymph sampling with microdialysis.
Another limitation of our study is the use of the hyperinsulinemic euglycemic clamp. While this is the gold standard for assessing insulin sensitivity in vivo, there are limitations to its physiological relevance, as an individual is normally not exposed to a sustained, supraphysiological level of insulin. Thus, assessing insulin access under these conditions may not be representative of the shorter-term elevations that occur when an animal is exposed to a glucose challenge, and further studies investigating insulin access under a more dynamic glucose and insulin environment, such as an intravenous or oral glucose tolerance test, may clarify whether impaired insulin access occurs in a physiological setting. However, under these high arterial insulin levels, the vein insulin concentrations are also high, and insulin levels are slow to respond, and much lower than plasma levels.
In conclusion, we demonstrate that obesity induced by a diet high in polyunsaturated fatty acids is not associated with the development of insulin resistance or impairments in insulin access to skeletal muscle. We confirm that the insulin level in the interstitial space in animals is approximately half that of plasma, suggesting a barrier to insulin transport across the endothelium that appears to be exacerbated by a saturated fat, but not polyunsaturated fat, diet. Further research is needed to determine whether dietary supplementation with polyunsaturated fat is protective against insulin resistance, plasma hyperinsulinemia, reduced insulin clearance and impaired insulin access to muscle associated with saturated fat diets.
Study Importance.
Insulin must access the skeletal muscle interstitium to cause glucose uptake
Saturated high fat diet-induced obesity is associated with insulin resistance and impaired insulin access to muscle at low insulin levels.
We show that a polyunsaturated high fat diet induces obesity, but not insulin resistance or impaired insulin access, thus distinguishing the effects of adiposity and insulin resistance on insulin access.
Acknowledgments
The authors would like to thank Rita Thomas, CSMC, for performing the insulin, and the Cedars-Sinai Medical Center Comparative Medicine staff for their assistance with and care for our animals. C.M.K. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Funding: This work was supported by NIH grants DK29867 and DK27619 and Society in Science, The Branco Weiss Fellowship, administered by the ETH Zürich (to JLB).
Footnotes
The authors have no potential conflicts of interest relevant to this article, but CMK and RNB have a grant from AstraZeneca, outside the submitted work.
Author contributions: JLB and CMK conceived and carried out the experiments, and analyzed data. IAB, RLP and MSI carried out experiments. All authors were involved in writing the paper and had final approval of the manuscript.
References
- 1.Okon EB, Chung AW, Zhang H, Laher I, van Breemen C. Hyperglycemia and hyperlipidemia are associated with endothelial dysfunction during the development of type 2 diabetes. Can J Physiol Pharmacol. 2007;85:562–567. doi: 10.1139/y07-026. [DOI] [PubMed] [Google Scholar]
- 2.Schaefer C, Biermann T, Schroeder M, et al. Early microvascular complications of prediabetes in mice with impaired glucose tolerance and dyslipidemia. Acta Diabetol. 2009;47:19–27. doi: 10.1007/s00592-009-0114-7. [DOI] [PubMed] [Google Scholar]
- 3.Czernichow S, Greenfield JR, Galan P, et al. Microvascular dysfunction in healthy insulin-sensitive overweight individuals. J Hypertens. 2010;28:325–332. doi: 10.1097/HJH.0b013e328333d1fc. [DOI] [PubMed] [Google Scholar]
- 4.Dell’Omo G, Penno G, Pucci L, et al. Abnormal capillary permeability and endothelial dysfunction in hypertension with comorbid Metabolic Syndrome. Atherosclerosis. 2004;172:383–389. doi: 10.1016/j.atherosclerosis.2003.11.013. [DOI] [PubMed] [Google Scholar]
- 5.Eringa EC, Serne EH, Meijer RI, et al. Endothelial dysfunction in (pre)diabetes: characteristics, causative mechanisms and pathogenic role in type 2 diabetes. Rev Endocr Metab Disord. 2013;14:39–48. doi: 10.1007/s11154-013-9239-7. [DOI] [PubMed] [Google Scholar]
- 6.Goldfine AB, Beckman JA, Betensky RA, et al. Family history of diabetes is a major determinant of endothelial function. J Am Coll Cardiol. 2006;47:2456–2461. doi: 10.1016/j.jacc.2006.02.045. %20. [DOI] [PubMed] [Google Scholar]
- 7.Castillo C, Bogardus C, Bergman R, Thuillez P, Lillioja S. Interstitial insulin concentrations determine glucose uptake rates but not insulin resistance in lean and obese men. J Clin Invest. 1994;93:10–16. doi: 10.1172/JCI116932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang YJ, Hope ID, Ader M, Bergman RN. Insulin transport across capillaries is rate limiting for insulin action in dogs. J Clin Invest. 1989;84:1620–1628. doi: 10.1172/JCI114339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sandqvist M, Strindberg L, Schmelz M, Lonnroth P, Jansson PA. Impaired delivery of insulin to adipose tissue and skeletal muscle in obese women with postprandial hyperglycemia. J Clin Endocrinol Metab. 2011;96:E1320–E1324. doi: 10.1210/jc.2011-0233. [DOI] [PubMed] [Google Scholar]
- 10.Sjostrand M, Gudbjornsdottir S, Holmang A, et al. Delayed transcapillary transport of insulin to muscle interstitial fluid in obese subjects. Diabetes. 2002;51:2742–2748. doi: 10.2337/diabetes.51.9.2742. [DOI] [PubMed] [Google Scholar]
- 11.Broussard JL, Castro AVB, Iyer MS, et al. Insulin access to skeletal muscle is impaired during the early stages of diet-induced obesity. Obesity (Silver Spring) 2016 doi: 10.1002/oby.21562. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Symons JD, Abel ED. Lipotoxicity contributes to endothelial dysfunction: a focus on the contribution from ceramide. Rev Endocr Metab Disord. 2013;14:59–68. doi: 10.1007/s11154-012-9235-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carter JR, Schwartz CE, Yang H, Joyner MJ. Fish oil and neurovascular control in humans. Am J Physiol Heart Circ Physiol. 2012;303:H450–H456. doi: 10.1152/ajpheart.00353.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yamakawa K, Shimabukuro M, Higa N, et al. Eicosapentaenoic Acid supplementation changes Fatty Acid composition and corrects endothelial dysfunction in hyperlipidemic patients. Cardiol Res Pract. 2012;2012:754181. doi: 10.1155/2012/754181. Epub;%2012 Dec 26.: 754181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Broussard JL, Nelson MD, Kolka CM, et al. Rapid development of cardiac dysfunction in a canine model of insulin resistance and moderate obesity. Diabetologia. 2015 doi: 10.1007/s00125-015-3767-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ikomi F, Hunt J, Hanna G, Schmid-Schonbein GW. Interstitial fluid, plasma protein, colloid, and leukocyte uptake into initial lymphatics. J Appl Physiol (1985 ) 1996;81:2060–2067. doi: 10.1152/jappl.1996.81.5.2060. [DOI] [PubMed] [Google Scholar]
- 17.Bergman RN. Use of the canine model in aging-related metabolic research. 2010 [Google Scholar]
- 18.Bergman RN, Kim SP, Hsu IR, et al. Abdominal obesity: role in the pathophysiology of metabolic disease and cardiovascular risk. Am J Med. 2007;120:S3–S8. doi: 10.1016/j.amjmed.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 19.Castro AV, Woolcott OO, Iyer MS, et al. Increase in visceral fat Per Se does not induce insulin resistance in the canine model. Obesity (Silver Spring) 2014:10. doi: 10.1002/oby.20906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mittelman SD, Van Citters GW, Kim SP, et al. Longitudinal compensation for fat-induced insulin resistance includes reduced insulin clearance and enhanced beta-cell response. Diabetes. 2000;49:2116–2125. doi: 10.2337/diabetes.49.12.2116. [DOI] [PubMed] [Google Scholar]
- 21.de Jongh RT, Serne EH, Ijzerman RG, de Vries G, Stehouwer CD. Free fatty acid levels modulate microvascular function: relevance for obesity-associated insulin resistance, hypertension, and microangiopathy. Diabetes. 2004;53:2873–2882. doi: 10.2337/diabetes.53.11.2873. [DOI] [PubMed] [Google Scholar]
- 22.Lamping KG, Nuno DW, Coppey LJ, et al. Modification of high saturated fat diet with n-3 polyunsaturated fat improves glucose intolerance and vascular dysfunction. Diabetes Obes Metab. 2013;15:144–152. doi: 10.1111/dom.12004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu Y, Zhang C, Dong Y, et al. Activation of the AMP-activated protein kinase by eicosapentaenoic acid (EPA, 20:5 n-3) improves endothelial function in vivo. PLoS One. 2012;7:e35508. doi: 10.1371/journal.pone.0035508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu J, Jahn LA, Fowler DE, et al. Free fatty acids induce insulin resistance in both cardiac and skeletal muscle microvasculature in humans. J Clin Endocrinol Metab. 2011;96:438–446. doi: 10.1210/jc.2010-1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim SP, Catalano KJ, Hsu IR, et al. Nocturnal free fatty acids are uniquely elevated in the longitudinal development of diet-induced insulin resistance and hyperinsulinemia. Am J Physiol Endocrinol Metab. 2007;292:E1590–E1598. doi: 10.1152/ajpendo.00669.2006. [DOI] [PubMed] [Google Scholar]
- 26.Lanza IR, Blachnio-Zabielska A, Johnson ML, et al. Influence of Fish Oil on Skeletal Muscle Mitochondrial Energetics and Lipid Metabolites during High-Fat Diet. Am J Physiol Endocrinol Metab. 2013;304(12):E1391–E1403. doi: 10.1152/ajpendo.00584.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Villalba JM, Lopez-Dominguez JA, Chen Y, et al. The influence of dietary fat source on liver and skeletal muscle mitochondrial modifications and lifespan changes in calorie-restricted mice. Biogerontology. 2015;16:655–670. doi: 10.1007/s10522-015-9572-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bergman RN, Ader M, Huecking K, Van Citters G. Accurate assessment of beta-cell function: the hyperbolic correction. Diabetes. 2002;51(Suppl 1):S212–20. doi: 10.2337/diabetes.51.2007.s212. [DOI] [PubMed] [Google Scholar]
- 29.Wiig H, Swartz MA. Interstitial fluid and lymph formation and transport: physiological regulation and roles in inflammation and cancer. Physiol Rev. 2012;92:1005–1060. doi: 10.1152/physrev.00037.2011. [DOI] [PubMed] [Google Scholar]
- 30.Zawieja SD, Wang W, Wu X, et al. Impairments in the intrinsic contractility of mesenteric collecting lymphatics in a rat model of metabolic syndrome. Am J Physiol Heart Circ Physiol. 2012;302:H643–H653. doi: 10.1152/ajpheart.00606.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chakraborty S, Zawieja S, Wang W, Zawieja DC, Muthuchamy M. Lymphatic system: a vital link between metabolic syndrome and inflammation. Ann N Y Acad Sci. 2010;1207(Suppl 1):E94–102. doi: 10.1111/j.1749-6632.2010.05752.x. E94–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scallan JP, Hill MA, Davis MJ. Lymphatic vascular integrity is disrupted in type 2 diabetes due to impaired nitric oxide signalling. Cardiovasc Res. 2015;107:89–97. doi: 10.1093/cvr/cvv117. [DOI] [PMC free article] [PubMed] [Google Scholar]