Hepatocellular carcinoma (HCC) is a major global health problem.1 HCC is the sixth most common incident cancer and the fourth leading cause of cancer death in 2015.2 HCC is more common in men; 1 in 45 men and 1 in 113 women develop liver cancer before age 79 years in the world.2 HCC is a highly lethal cancer as a majority of patents with HCC are diagnosed in non-curative stages.3 While patients with early stage HCC are eligible for potentially curative treatment, up to 70% of patients who receive curative resection or local ablation eventually develop recurrent tumors within 5 years.1 There are no effective chemopreventive strategies for these patients and those at risk for HCC development.
In order to decrease the burden of HCC mortality, it is crucial to identify high risk groups of patients and provide interventions to decrease the risk of tumor development and recurrence after curative treatment. Prognostic biomarkers should provide information to identify such high risk patients. Serum alfa-fetoprotein (AFP) is a well-established diagnostic and prognostic biomarker for HCC.4 Although highly elevated serum AFP predicts a poor clinical outcome, therapeutic implications of an elevated AFP in HCC patients remain unclear. Similarly, several other proteomic or genomic biomarkers aid in diagnosis and correlate with prognosis; however, remain underutilized due to lack of wide availability. For example, with recent technical advances in genomics and transcriptomics, researchers used genome-wide expression profiling of liver cancer and adjacent benign tissues and showed that the gene expression pattern of tissue adjacent to the tumor predicts clinical outcome in HCC patients after surgical resection.5 While this gene expression signature provides prognostic information, it does not provide biologic information amenable to interventions which may improve clinical outcomes. More recently, sorafenib was tested as an adjuvant treatment but failed to prolong recurrent free survival following curative surgical resection or local ablation in a large phase 3, double-blind, placebo-controlled trial.6 Identification of mechanism-based prognostic biomarkers that can be intervened to improve the clinical outcomes is a critical unmet need in HCC research.
In the current issue of Hepatology, Fu et al have published their research examining the utility of an exocyclic deoxyguanosine DNA adduct, γ-OHPdG in 3 mouse models of hepatocarcinogenesis and its utility as a prognostic biomarker with further validation in human HCC samples. γ-OHPdG is one of the most common lipid peroxidation-derived DNA adducts, that can modify DNA bases including p53 genes and it frequently causes G to T and G to A mutations. It is a well-established mutagen in smoking-induced lung cancer. However, the role of γ-OHPdG in HCC has not been investigated in the past. The authors showed that γ-OHPdG levels increase with age in nucleotide excision repair deficient mice (Xpa−/−) and the whole liver levels correlated with HCC development. With whole exome sequencing of tumors and adjacent benign tissues, they determined that GC>TA mutation is the dominant alteration in Xpa−/− mice, implying that γ-OHPdG may play a role in HCC-specific mutagenesis. Next, authors showed that suppression of γ-OHPdG formation in the mouse liver led to inhibition of hepatocarcinogenesis. Three antioxidants (theaphenon E, α-lipoic acid, and α-tocopherol) were tested. Theaphenon E, which is a potent anti-oxidative green tea extract, was shown to lower the level of γ-OHPdG the most. Furthermore, theaphenon E prevented tumor formation and decreased tumor number and size in DEN-injected mice (Figure 1). Lastly, the authors examined the levels of γ-OHPdG in HCC patients who had undergone a liver resection and stratified them by γ-OHPdG expression level. Higher levels of γ-OHPdG were strongly associated with shorter overall survival and recurrence-free survival, confirming the role of γ-OHPdG as a prognostic biomarker in human HCC.
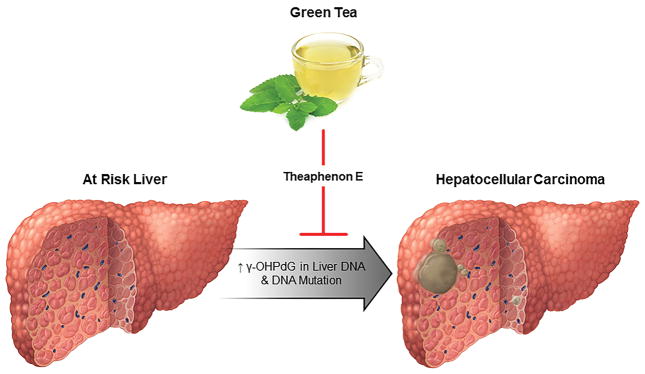
Figure 1. Green tea for the chemoprevention of hepatocellular carcinoma.
Gamma-hydroxy-1,N2-propanodeoxyguanosine (γ-OHPdG), a mutagenic lipid peroxidation-induced DNA adduct correlates with hepatocellular carcinoma (HCC) incidence in experimental murine models of HCC. Theaphenon E administration reduced the HCC tumor burden and γ-OHPdG levels, suggesting that the chemopreventive effect of green tea might be mediated by Theaphenon E by decreasing γ-OHPdG in liver DNA and consequent DNA mutations
The current study findings provide biologic evidence of the chemopreventive effect of theaphenon E for HCC. A population-based case-control study from China on 204 HCC cases and 415 healthy controls showed that green tea consumption was inversely associated with the risk of HCC. Individuals who drank green tea longer than 30 years were at lowest risk compared with non-drinkers (adjusted odds ratio of 0.44, 95% confidence interval [CI]: 0.19–0.96).7 A recent meta-analysis of 9 prospective cohort studies (465,274 subjects and 3694 cases) again confirmed the association between green tea consumption and reduced risk for liver cancer (relative risk, 0.88, 95% CI: 0.19–0.96).8 While the vast majority of epidemiologic evidence supporting the protective effect of green tea is from Asia, a recent European study confirmed a preventative effect of tea on HCC: tea intake was associated with a 59% reduction in the risk of HCC development among 486,799 subjects after a median follow-up of 11 years.9 However type of tea was not available in that European study.
An important limitation of the current study is the small number of human HCC tissues analyzed. It remains unclear whether the γ-OHPdG levels are independently associated with clinical outcomes after adjusting for known prognostic factors such as tumor size, number of lesions, AFP, vascular invasion and metastasis. For the same reason, it has not been investigated whether γ-OHPdG is implicated in hepatocarcinogenesis in an etiology-specific manner. Nonetheless, the authors reported a mechanistically relevant prognostic biomarker that can help select a subgroup of HCC patients who may benefit from theaphenon E/green tea consumption.
Should we recommend green tea consumption in patients who are at increased risk for HCC development or recurrence? The current study provides a biologic mechanism for the protective effect of green tea on HCC, which has been demonstrated in previous epidemiological studies. Therefore, we may encourage high risk patients to drink green tea. In order to clearly measure the biologic impact of theaphenon E/green tea, association between green tea consumption, the level of γ-OHPdG in tumor and adjacent benign tissues DNA and HCC development and recurrence should be externally validated especially in western countries where it has been under studied. Lastly, it is worth mentioning that green tea catechins exert chemopreventive properties by modulating signal transduction and metabolic pathways, hence inhibiting metabolic syndrome-related carcinogenesis in addition to antioxidant and anti-inflammatory activities.10 This might provide a different level of preventive effect in nonalcoholic steatohepatitis, which is emerging as a major etiology of HCC. Etiology-specific mutagenic role of γ-OHPdG in hepatocarcinogenesis should be further investigated in the future and this will help identifying patients who may get the most benefit from an anti-oxidant effect of green tea in the era of precision and individualized medicine.
Acknowledgments
This work was supported in part by the Mayo Foundation and the National Institutes of Health grants DK107402 and DK111378 (to H.M.).
Footnotes
Conflict of Interest: Nothing to disclose
References
- 1.Yang JD, Roberts LR. Hepatocellular carcinoma: A global view. Nat Rev Gastroenterol Hepatol. 2010;7:448–58. doi: 10.1038/nrgastro.2010.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fitzmaurice C, Allen C, Barber RM, et al. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-years for 32 Cancer Groups, 1990 to 2015: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2016 doi: 10.1001/jamaoncol.2016.5688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang JD, Ahmed Mohammed H, Harmsen WS, et al. Recent Trends in the Epidemiology of Hepatocellular Carcinoma in Olmsted County, Minnesota: A US Population-based Study. J Clin Gastroenterol. 2017 doi: 10.1097/MCG.0000000000000810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang JD, Kim WR, Park KW, et al. Model to estimate survival in ambulatory patients with hepatocellular carcinoma. Hepatology. 2012;56:614–21. doi: 10.1002/hep.25680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoshida Y, Villanueva A, Kobayashi M, et al. Gene expression in fixed tissues and outcome in hepatocellular carcinoma. N Engl J Med. 2008;359:1995–2004. doi: 10.1056/NEJMoa0804525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bruix J, Takayama T, Mazzaferro V, et al. Adjuvant sorafenib for hepatocellular carcinoma after resection or ablation (STORM): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2015;16:1344–54. doi: 10.1016/S1470-2045(15)00198-9. [DOI] [PubMed] [Google Scholar]
- 7.Li Y, Chang SC, Goldstein BY, et al. Green tea consumption, inflammation and the risk of primary hepatocellular carcinoma in a Chinese population. Cancer Epidemiol. 2011;35:362–8. doi: 10.1016/j.canep.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang YQ, Lu X, Min H, et al. Green tea and liver cancer risk: A meta-analysis of prospective cohort studies in Asian populations. Nutrition. 2016;32:3–8. doi: 10.1016/j.nut.2015.05.021. [DOI] [PubMed] [Google Scholar]
- 9.Bamia C, Lagiou P, Jenab M, et al. Coffee, tea and decaffeinated coffee in relation to hepatocellular carcinoma in a European population: multicentre, prospective cohort study. Int J Cancer. 2015;136:1899–908. doi: 10.1002/ijc.29214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shimizu M, Shirakami Y, Sakai H, et al. Chemopreventive potential of green tea catechins in hepatocellular carcinoma. Int J Mol Sci. 2015;16:6124–39. doi: 10.3390/ijms16036124. [DOI] [PMC free article] [PubMed] [Google Scholar]