Fig. 1. Proposed biosynthesis and transport of A. baumannii lipooligosaccharide (LOS).
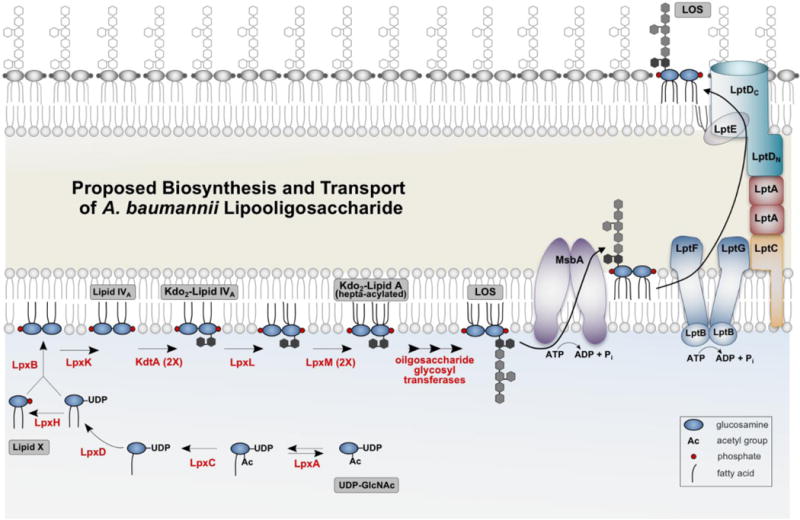
Typically, the Kdo2-lipid A domain of LPS or LOS is required for growth of gram-negative bacteria. The A. baumannii genome contains homologs of the necessary Lpx enzymes to synthesize the Kdo2-lipid A substructure similar to that of E. coli, although the Acinetobacter enzymes have not been fully characterized. Each step of Kdo2-lipid synthesis requires a single enzyme (red) with the early steps of the pathway catalyzed by soluble enzymes and the latter steps catalyzed by membrane associated proteins. Each acyl transferase has a preferred acyl chain specificity that is dictated by an active site hydrocarbon ruler and acyl-ACP (acyl carrier protein) serves as the acyl donor. Although the Kdo sugars are part of the inner core-oligosaccharide, in some organisms Kdo addition is required for lipid A biosynthesis as the final two steps, catalyzed by LpxL and LpxM, require the presence of covalently attached Kdo. In E. coli, both LpxL and LpxM catalyze the addition of a single acyl chain producing the final hexa-acylated lipid A species. However, A. baumannii LpxM catalyzes the addition of two acyl chains resulting in an hepta-acylated lipid A structure (Boll et al., 2015). The remaining sugars of the oligosaccharide of LOS are extended on the cytoplasmic leaflet of the inner membrane prior to transport by the ABC transporter MsbA that flips A. baumannii LOS to the periplasmic face of the inner membrane. Although absent in Acinetobacter, for organisms with a complete LPS structure, the O-antigen domain is added on the periplasmic face of the inner membrane. Finally, the intermembrane transport of LOS is performed by the Lpt (LPS transport) proteins that comprise an envelope-spanning translocation machine.
