Fig. 2. Comparison of lipid A structures of E. coli and A. baumannii.
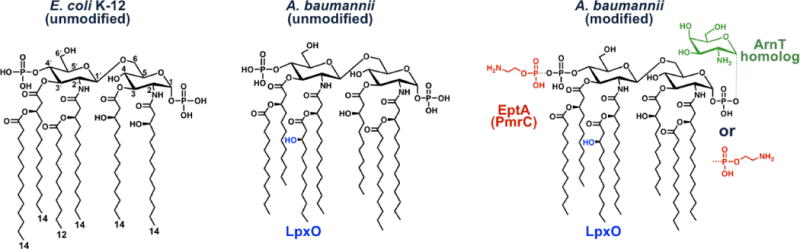
The predominant lipid A species of E. coli K-12 is hexa-acylated and phosphorylated at the 1- and 4′-positions. A. baumannii is similar to that of E. coli except an additional acyl chain is found in an acyloxyacyl-linkage at the 2-position arising from a second acylation event catalyzed by LpxM (Figure 1). Wildtype A. baumannii lipid A also contains an additional hydroxyl group on the 2´-linked secondary acyl chain that likely arises from the action of an LpxO homolog found in the A. baumannii genome (Boll et al., 2015). In colistin resistant A. baumannii that maintain LOS, the lipid A phosphate groups can be modified with galactosamine and with phosphoethanolamine residues. Phosphoethanolamine modification can occur at both phosphates (Arroyo et al., 2011), whereas only single modified species of galactosamine have been reported (Pelletier et al., 2013) thus far. EptA (PmrC) catalyzes the addition of phosphoethanolamine in A. baumannii (Arroyo et al., 2011) and galactosamine addition is likely catalyzed by an A. baumannii ArnT homolog. In Salmonella and E. coli, ArnT transfers the sugar L-4-aminoarabinose to lipid A phosphate groups (Needham and Trent, 2013).
