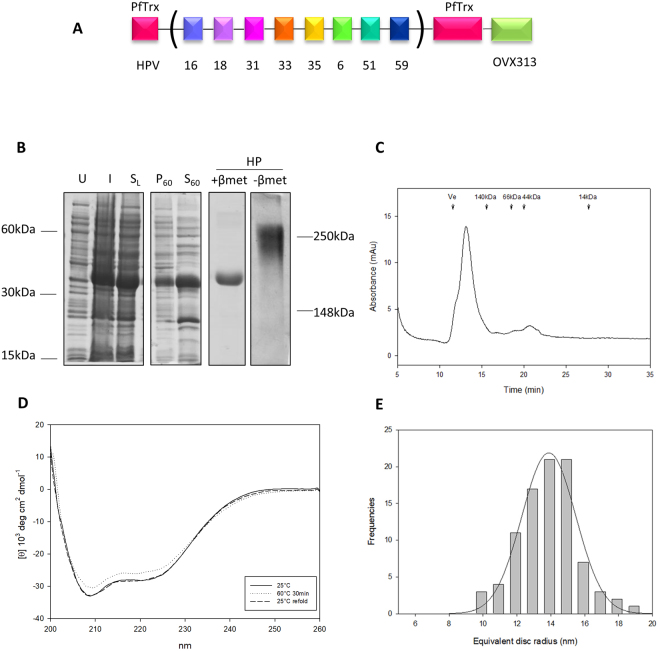
Figure 5.
Design, purification and biochemical characterization of the multiepitope PfTrx-L2(8x)-OVX313 antigen. (A) Scheme of the PfTrx-L2(8x)-OVX313 construct (see Fig. S1 for the polypeptide sequence and Fig. S5 for the rationale underlying the choice of this particular set of L2 epitopes). (B) SDS-PAGE analysis under reducing conditions (+β-mercaptoethanol; βmet) of the total lysate from uninduced (U) and IPTG-induced (I) E. coli cells overexpressing the PfTrx-L2(8x)-OVX313 antigen, and of the resulting soluble lysate fraction (SL). The pelletable/heat-denatured (P 60) and the soluble/heat-stable (S 60) fractions recovered after heat-treatment of the soluble lysate for 30 min at 60 °C followed by centrifugation at 8,000 x g for 15 min are shown in the fourth and the fifth lane, respectively. The pool of salt-eluted, heparin-purified (HP) fractions electrophoresed under reducing (+βmet) or non-reducing (−βmet) conditions is shown in the last two lanes. Lanes U, I and FT, plus P 60 and S 60 were cropped from a single gel (see Fig. S7C for a full-length gel image); lanes labeled HP represent the results of single-sample SDS-PAGE analyses separately performed on the post-heparin pool. (C) Size exclusion chromatography analysis of the heparin-purified PfTrx-L2(8x)-OVX313 antigen; Ve, excluded volume (see ‘Methods’ for details). (D) Far-UV circular dichroism analysis of PfTrx-L2(8x)-OVX313 thermal stability carried out after heat-treatment at 60 °C for 30 min (dashed line) and after heat-treatment followed by a 10 min recovery at 25 °C (dotted line); the antigen kept at 25 °C served as an untreated sample control (solid line). (E) Equivalent disc radius distribution of the PfTrx-L2(8x)-OVX313 nanoparticles determined by AFM.