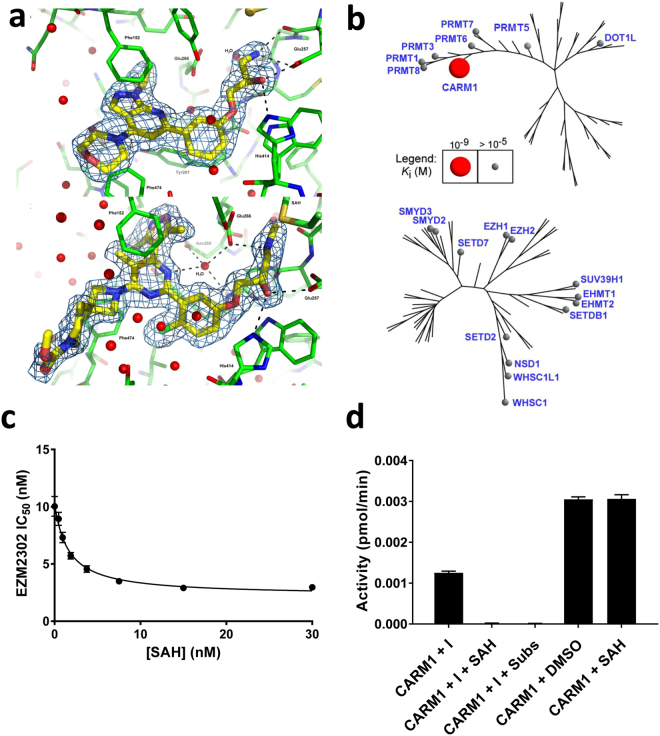
Figure 2.
EZM2302 binds to CARM1 and is a selective inhibitor of CARM1 activity. (a) Structure of 2 (top) or EZM2302 (bottom) (yellow) in complex with CARM1 (green). Electron density (2Fo-Fc, 1σ for the compound is shown. Hydrogen bonds are indicated as black dashes; Π interactions are indicated with orange dashes; water molecules are depicted as spheres. (b) Ligand affinity maps of EZM2302 across the family trees of human arginine methyltransferases and lysine methyltransferase enzymes show EZM2302 is a selective and potent inhibitor of CARM1. (c) Synergy of CARM1 inhibition by EZM2302 with SAH. IC50 values for EZM2302 were determined at increasing concentrations of SAH. Data were fit using the noncompetitive Cheng-Prusoff and the Yonetani-Theorell equations as shown in the Supplementary Methods. Potency of inhibition by EZM2302 increases with SAH concentrations. (d) EZM2302 inhibition of CARM1. CARM1 was preincubated with excess EZM2302 (I) in the presence and absence of SAH and substrates (Subs). The CARM1 complexes were purified by gel filtration using 0.5 mL 7 K MWCO Zeba columns (Peirce) and CARM1 activity was tested.