Figure 7.
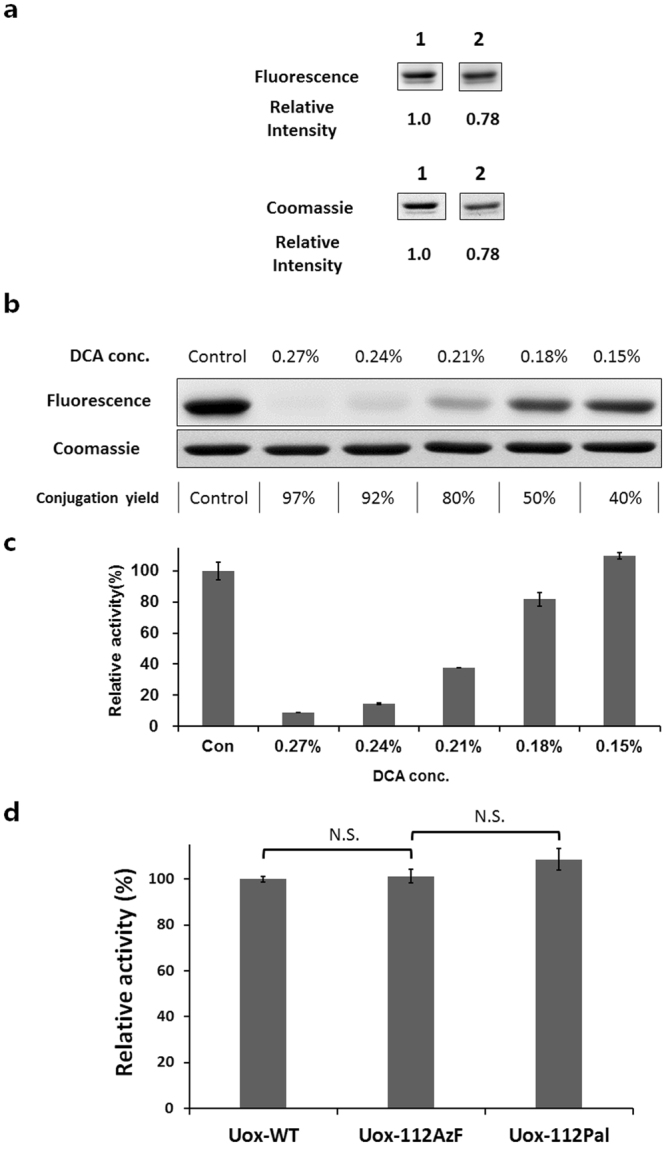
Effects of deoxycholic acid (DCA) addition on the conjugation yield of DBCO-Pal to Uox-112AzF. (a) Fluorescence and Coomassie-stained images of protein gels of the 50 μM Uox-112AzF samples incubated without (lane 1) or with (lane 2) 100 μM DBCO-Pal for 2 hr followed by reaction with 120 μM DBCO-MB543 fluorescence dye for 2 hr. Relative intensity values indicate the band intensities of Uox-112AzF samples incubated with DBCO-Pal relative to that of Uox-112AzF samples incubated without DBCO-Pal. These are cropped gel parts and the full-length gel images are shown in Fig. S5a. (b) Fluorescence and Coomassie-stained images of protein gels of the 50 μM Uox-112AzF samples reacted with 100 μM DBCO-Pal for 2 hr at varying concentrations of DCA (0, 0.15, 0.18, 0.21, 0.24, and 0.27 %) followed by reaction with 120 μM DBCO-MB543 fluorescence dye for 2 hr. Conjugation yields were calculated by the ratio of the fluorescence intensity of the sample prepared in the presence of DCA over that of the sample prepared in the absence of DCA. These are cropped gel parts and the full-length gel images are shown in Fig. S5b. (c) Enzymatic activity (100 μM uric acid) of the reaction mixture of Uox-112AzF (50 μM) and DBCO-Pal prepared at varying concentrations of DCA relative to that of the reaction mixture prepared in the absence of DCA. After the conjugation, the reaction mixture was desalted prior to enzymatic activity assays. Error bars represent standard deviations (n = 3). (d) Enzymatic activities of Uox-112AzF and Uox-Pal relative to that of Uox-WT (50 nM Uox variant, 100 μM uric acid, 30 min). Error bars represent standard deviations (n = 3). Enzymatic activity was not significantly different after incorporation of AzF and Pal conjugation. Enzymatic activities of Uox-112AzF and Uox-Pal were retained (two-tailed student’s t-test; N.S. indicates p > 0.01).
