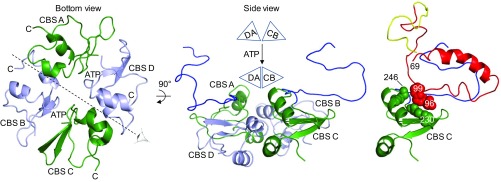
Fig. 6.
How ATP-induced movement of the SpoIVFB CBS domain tetramer might position Pro-σK for cleavage. (Left) The bottom view is the same as in Fig. 5A except the interdomain linkers and the Pro-σK molecules have been removed. CBS domains are labeled according to their chain, and each C terminus is indicated to highlight the antiparallel arrangement. The eye and dashed line indicate the axis of the side view shown in Middle. In the side view, the A and D chain CBS pair is on the left with CBS D in front. The pair is represented as a triangle labeled DA in the schematic above. The C and B chain CBS pair (represented as triangle CB above) is on the right with CBS C in front. The A and C chain linkers are in blue. The schematic illustrates ATP-induced movement of the CBS domain pairs, which would cause the A and C chain linkers to move. Right shows the interaction of Pro-σK chain Y with the linker and CBS domain of chain C. The interaction is proposed to allow movement of the C and B chain CBS pair to position Pro-σK for cleavage. Residues 70–95 and 100 to the C terminus of Pro-σK have been removed so that interaction of residues 28–69 with the SpoIVFB linker can be seen (see also SI Appendix, Fig. S10), and residues 96–99 of Pro-σK (red space-filling, main chain only) as well as residues 228–230, 241, and 246 of CBS C (green space-filling, main chain only) are shown to highlight their interaction (see also SI Appendix, Fig. S11).