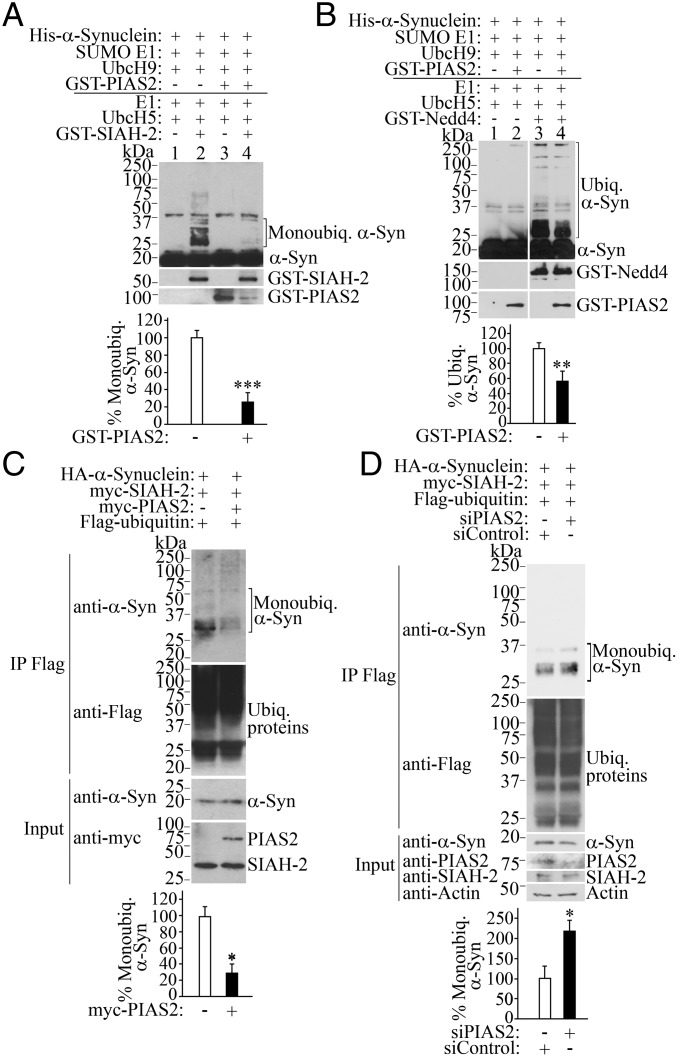
Fig. 2.
SUMOylation by PIAS2 prevents α-synuclein monoubiquitination. (A) Inhibition of SIAH-2–mediated monoubiquitination by PIAS2. In vitro SUMOylation/ubiquitination reactions were carried out by incubating His–α-synuclein with purified SUMOylation components for 60 min, followed by the addition of ubiquitination components for another 60 min. Levels of His–α-synuclein monoubiquitination were determined with anti–α-synuclein. Graph shows the percentage of SIAH-2–dependent α-synuclein monoubiquitination, with and without GST-PIAS2 by comparing lanes 2 and 4. (B) PIAS2 inhibits Nedd4-mediated α-synuclein ubiquitination. Conditions were the same as in A. Graph depicts the percentage of Nedd4-dependent α-synuclein ubiquitination in the absence and in the presence of GST-PIAS2. (C) PIAS2 decreases α-synuclein monoubiquitination in cells. Ubiquitinated proteins from transfected HEK293 cells were immunoprecipitated with anti-Flag antibody, and monoubiquitinated α-synuclein was detected with anti–α-synuclein (first panel). Graph depicts the percentage of monoubiquitinated α-synuclein in cells relative to unmodified α-synuclein. (D) HEK293 cells were transfected as indicated, and in the presence of siRNA control or PIAS2 siRNA. Immunoprecipitation was carried out and analyzed as in C. Graph represents the percentage of monoubiquitinated α-synuclein in cells relative to unmodified α-synuclein. Values represent the average ± SEM of three experiments. Different from control at *P < 0.05, **P < 0.01, and ***P < 0.001 (Student’s t test).