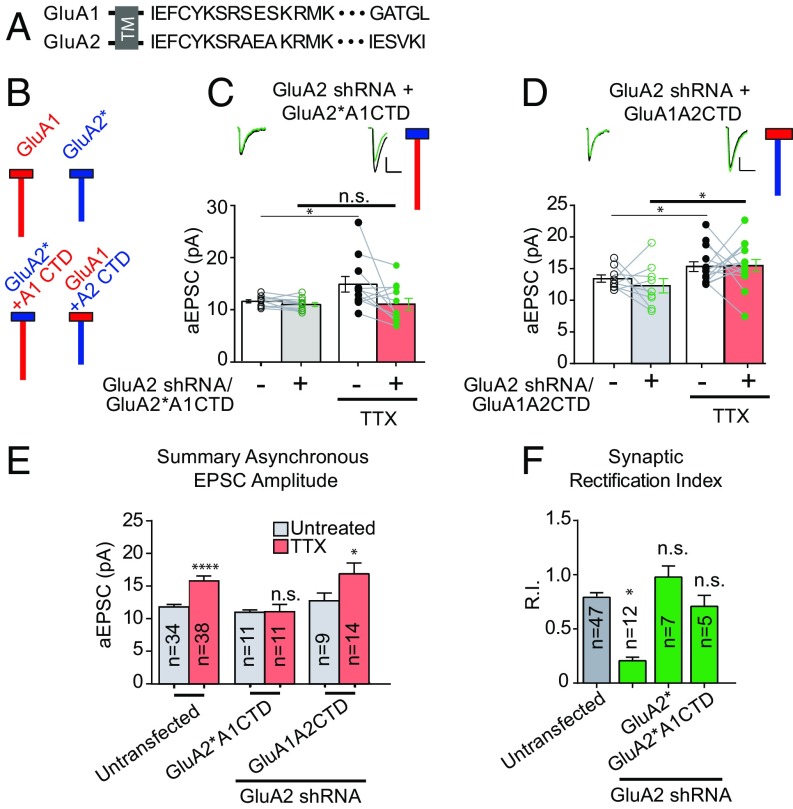
Fig. 3.
The C-tail of the GluA2 subunit is critical for homeostatic synaptic scaling. (A) Endogenous GluA1 and GluA2 C-tail amino acid sequences. TM, transmembrane. (B) Schematic diagram of endogenous GluA1 and GluA2 AMPAR subunits next to schemata of chimeric AMPARs with swapped C-tails. Red indicates GluA1 subunit origin; blue, GluA2 subunit origin. Boxes indicate amino terminal domains and transmembrane regions of AMPARs, and vertical lines indicate intracellular C-tails. (C) Paired asynchronous recordings without and with preceding chronic TTX treatment in control neurons and neighboring neurons transfected with GluA2 shRNA and shRNA-insensitive AMPAR chimeric subunit GluA2*A1CTD. (D) GluA2 shRNA + shRNA-insensitive AMPAR chimeric subunit GluA1A2CTD. Treatment conditions are the same as in C. (E) Summary bar graph indicating unpaired scaling data under the same transfection conditions. Significance was measured across treatment conditions. (F) Comparison of synaptic rectification of chimeric AMPAR GluA2*A1CTD (with pore residue conferring calcium and intracellular polyamine block present) with cells transfected with GluA2 shRNA for comparison and cells transfected with GluA2 shRNA + full-length shRNA-insensitive GluA2. (Scale bars for aEPSC sample traces: 5 pA and 20 ms unless indicated otherwise.) *P < 0.05; ****P < 0.0001; n.s., not significant.