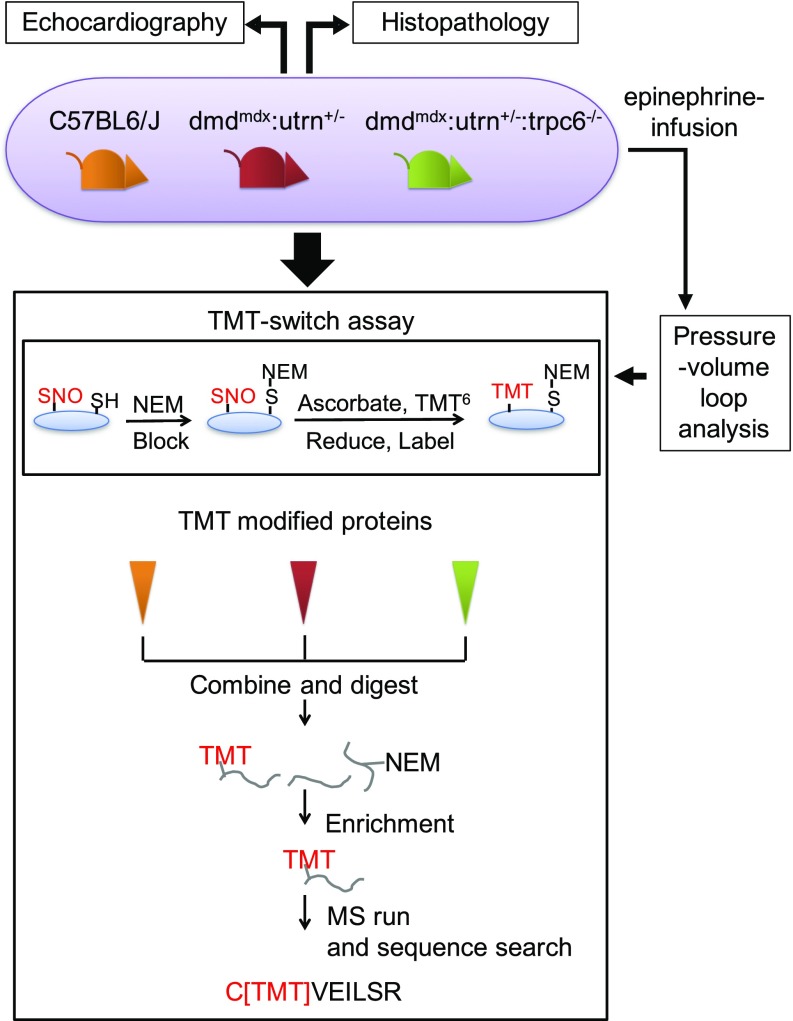
Fig. 1.
Multiple experimental approaches were employed to search for a linkage between Trpc6, nitrosative stress, and the pathobiology of DMD in the dmdmdx:utrn+/− mice. To capture protein S-nitrosylation, by an MS-coupled dual-labeling strategy, protein mouse heart lysates were labeled with multiplex cys- or iodoTMT6. Samples processed in the absence of ascorbate served as negative controls. Modified proteins were digested, enriched, desalted, and analyzed using MS. C[TMT]VEILSR, an example of TMT-labeled peptides; SH, free cysteine; S-NEM, cysteine blocked with N-ethylmaleimide (NEM).