TABLE A6.
Optional comments for laboratory test reportsa
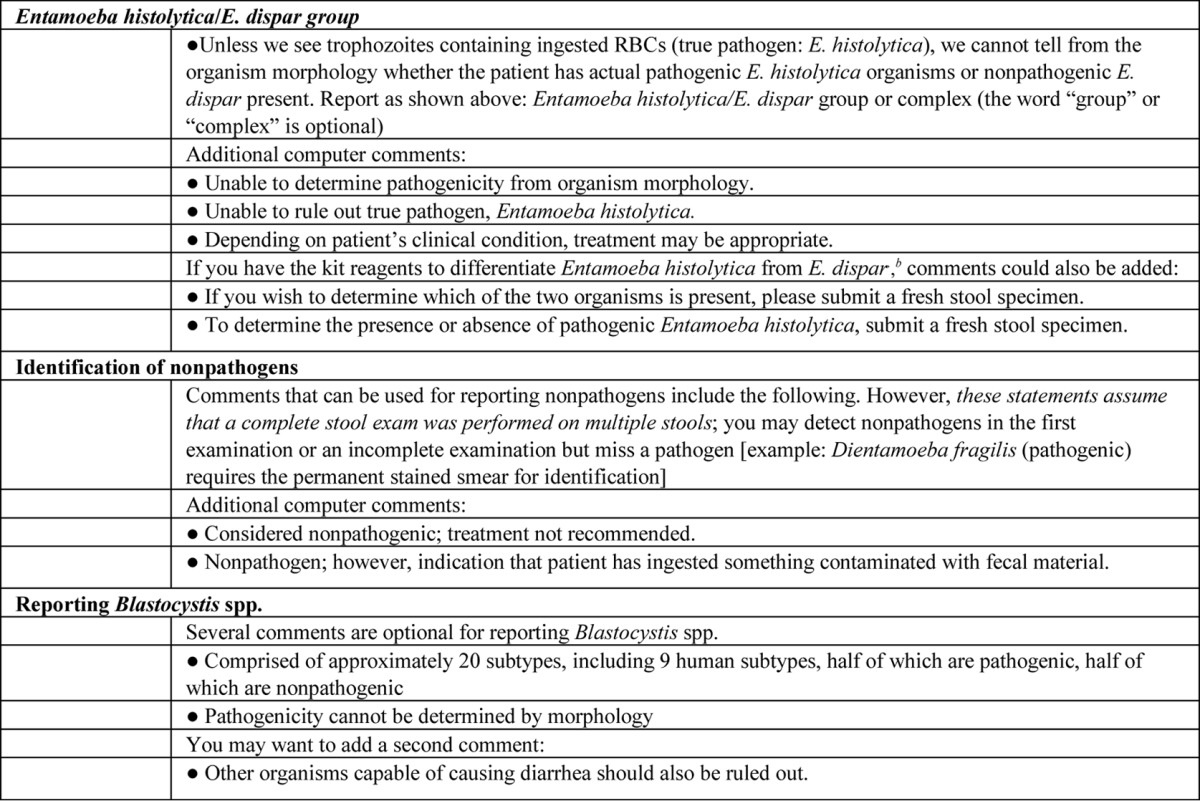
It is important to remember that educational information for your clients is critical to the success of your test reporting formats. The information in the table should be shared with your clients prior to changing your actual reporting formats. Your physician group may have a preference regarding additional comments. Information updates or newsletters are appropriate for this purpose. All of the comments in the table are optional, and wording can be changed to fit your circumstances. However, it is recommended that you select specific comments and try not to use “free text,” so that everyone reports test results in the same way each time. Adapted from reference 5.
It is important to remember that current fecal immunoassay kits for the detection of the Entamoeba histolytica/E. dispar group or for differentiation between the true pathogen (E. histolytica) and the nonpathogen (E. dispar) require fresh or frozen fecal specimens; although preserved specimens (generally preserved in a formalin-based fixative or some of the single-vial fixatives, universal fixative/no formalin/no mercury/no PVA/Total-Fix) can be used for the fecal immunoassays for Giardia lamblia or Cryptosporidium spp., they cannot be used for Entamoeba species testing.
