Abstract
Glial cells are essential for proper formation and maintenance of the nervous system. During development, glia keep neuronal cell numbers in check and ensure that mature neural circuits are appropriately sculpted by engulfing superfluous cells and projections. In the adult brain, glial cells offer metabolic sustenance and provide critical immune support in the face of acute and chronic challenges. Dysfunctional glial immune activity is believed to contribute to age-related cognitive decline, as well as neurodegenerative disease risk, but we still know surprisingly little about the specific molecular pathways that govern glia-neuron communication in the healthy or diseased brain. Drosophila offers a versatile in vivo model to explore the conserved molecular underpinnings of glial cell biology and glial cell contributions to brain function, health, and disease susceptibility. This review addresses recent findings describing how Drosophila glial cells influence neuronal activity in the adult fly brain to support optimal brain function and, importantly, highlights new insights into specific glial defects that may contribute to neuronal demise.
Introduction
Although it is becoming increasingly clear that glial cells are key players in nervous system development, plasticity, and homeostasis, we know little about the molecular pathways that underlie glia-neuron interactions in the developing and mature brain. The fruit fly Drosophila melanogaster is a powerful invertebrate organism model for investigating conserved molecular and cellular features of glia in the embryonic and adult CNS. Drosophila are affordable, easy to maintain, and offer unprecedented power to genetically manipulate and visualize cells in vivo, even at the single cell level. Moreover, our knowledge of Drosophila glial cell anatomy, gene expression, and function is growing exponentially. As in the mammalian brain, Drosophila glia can be parsed into discrete subtypes that perform unique functions, including CNS pruning, regulation of synaptic signaling, blood-brain barrier formation, and neuroprotection (1, 2). This review will not specifically explore the role of Drosophila glia during nervous system development as this topic has been addressed in a number of excellent recently published reviews (2–4). Instead, this article will highlight new findings regarding adult glial cell function, with a particular emphasis on brain homeostasis and new insights from Drosophila models exploring glia-neuron interactions in the context of injury and neurodegenerative disorders.
Glial diversity in adult Drosophila
The distribution and unique morphological classes of glial cells in the adult Drosophila brain has only been realized in the last decade (5–8). Currently, adult fly glia are classified into five major subtypes. Cortex glia envelop neuronal cell bodies throughout the cortical regions of the brain. Astrocytes extend highly branched projections into neuropil regions to functionally regulate neuronal signaling. Ensheathing glia enwrap axonal tracts throughout the central and peripheral nervous systems. Finally, subperineurial and perineurial glia comprise a double-layered glial sheath that forms a contiguous surface covering the entire CNS and PNS, which is often referred to as the Drosophila blood-brain barrier (BBB) (1, 9) (Figure 1). Ongoing work is providing critical new insight into the molecular and functional fingerprints of mature fly glia and similarities with their vertebrate counterparts. For example, a recent tour de force analysis, which characterized a large collection of novel in vivo genetic drivers, now offers more comprehensive information about the morphological diversity of glia, as well as cellular interactions in various brain regions, demonstrating, for example, that homotypic and heterotypic repulsion mechanisms maintain glial tiling in the adult CNS (10). In addition, a novel glial cell type (the “Semper” cone cell), which is strikingly similar to vertebrate Muller glia, has been identified in the Drosophila retina (11*) (Figure 1). Both Semper cells and Muller glia radially span the retina and express high levels of crystalline-like proteins to facilitate light scattering throughout retinal tissue. The genetic signatures of Semper cells and Muller glia overlap considerably, as both express critical genes, including inward rectifying potassium channels, Na/K-ATPase, excitatory amino acid transports (e.g. EAAT1), and glucose transporters (e.g. Glut1), to support photoreceptor energetics and signaling. Finally, newly published collections of transcriptional profiling experiments employing FACS sorting and in vivo transcript isolation strategies are now providing an unprecedented picture of the genetic profile of glial subtypes in the adult fly brain (11–14). Although glial cell biologists are just beginning to scratch the surface of resolving the varied functional roles of Drosophila glia, it is clear that emerging genetic toolkits and in vivo profiling strategies will rapidly advance our understanding of glial influences on neuronal function and brain health.
Figure 1. Glial subtypes in the adult Drosophila CNS.
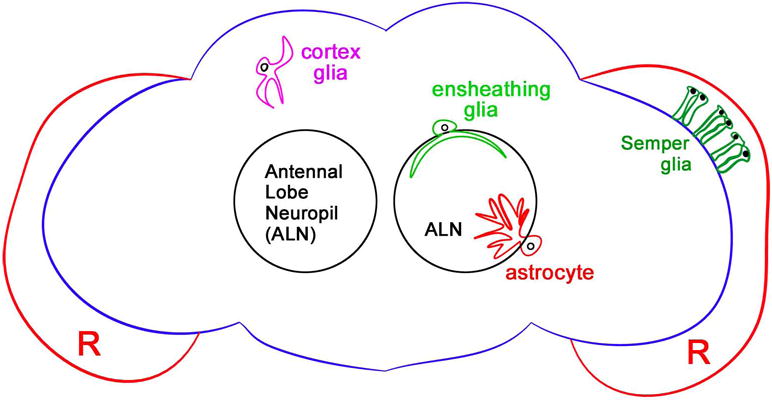
Schematic diagram of the adult fly brain illustrates representative classes of glia. In the cortical regions of the CNS, cortex glia (magenta) surround neuronal cell bodies, likely providing important metabolic and functional support. Neuropil areas of the central brain, which house axonal and dendritic projections, contain two major glial subtypes: Ensheathing glia (light green) enwrap neuronal extensions and appear to serve as the primary immune responders in adult animals, while astrocytes (red) modulate synaptic signaling. Representative diagrams of ensheathing glia and astrocytes within the antennal lobe neuropil (ALN) are shown. The entire nervous system is covered by a double layer of surface glial cells (blue outline), which offers a protective barrier between the CNS and the circulating hemolyph. A more recently identified cohort of glial cells in the adult retina (R), Semper cells (dark green), regulate photoreceptor function in a manner comparable to mammalian Mueller glia.
Glial support of metabolism and brain health
Glial cells perform the very important task of maintaining metabolic homeostasis in the nervous system. Glia-neuron metabolic coupling has been well documented in mammals, between myelinating glia and axons, as well as astrocytes and synapses (15–18), and recent work investigating metabolic regulation in Drosophila now highlights the evolutionary conservation of energy homeostatic mechanisms. In insects, including Drosophila, the sugar trehalose is the major energy metabolite that circulates within the hemolymph, the fluid that flows through the insect’s open circulatory system and bathes all organs. Until recently, it was unknown how trehalose and derived metabolites were transferred to the CNS. Volkenhoff and colleagues (2015) now show that Drosophila employ a “glia to neuron” shuttling system to provide these critical metabolic factors to neurons. The perineurial glial cells of the blood brain barrier specifically express the trehalose transporter Tret1-1 to absorb trehalose from the hemolymph (19). Within this subset of glia, trehalose is first broken down to glucose and then further metabolized to alanine or lactate, which can be taken up by local neurons to meet energy demands. Although the perineurial glia express ample glycolytic enzymes to break down trehalose, glycolytic gene expression is strikingly low in neurons, which renders neurons dependent on glia for precious energy sources. Notably, glial-specific knockdown of glycolysis results in neurodegeneration, while neuronal glycolysis is largely dispensible (19, 20). Looming questions still remain as to how metabolites are transferred from glia to neurons and, potentially, also between glial cells. It will also be important to determine if and how metabolite transfer occurs in an activity-dependent manner. Nonetheless, this metabolic compartmentalization between neurons and glia is clearly an evolutionarily conserved strategy, which underscores the importance of glial-neuron metabolic interplay and emphasizes the value of Drosophila as a powerful model to resolve the molecular details of CNS energy metabolism.
Glia in the context of healthy aging and acute injury
Age-related changes in glial cells and glial-neuron signaling networks profoundly influence CNS plasticity, cognition, and overall organismal function. Thus, scientists are eager to define the cellular and molecular changes that occur in senescent glia. Several recent studies have provided new insight into how changes in innate immune function contribute to brain aging, as well as susceptibility to neural damage and disease.
One highly conserved glial immune pathway includes Draper (known as MEGF10 in mammals), an engulfment receptor that is required in flies and vertebrates for proper glial clearance of degenerating neuronal projections, synapses, and apoptotic neurons during development (21–26). Draper couples to several downstream signaling pathways via tyrosine kinases, including the c-Jun N-terminal kinase (JNK) cascade, to alter cytoskeletal remodeling, gene transcription, and phagocytic function in glial cells (27–29). In Drosophila, Draper is also required for glial engulfment of degenerating axons after acute nerve axotomy in adults (23, 27, 29, 30). Notably, neurodegeneration occurs in aged draper mutant animals (31, 32), and this phenotype is rescued by glial expression of target of rapamycin 1 (TORC1), one factor implicated in proper processing of phagocytosed material (32). The neurodegenerative phenotype in draper mutants may arise, at least in part, from incomplete clearance of neuronal corpses during development. However, it is important to consider that dysfunctional glial engulfment (due to lack of Draper) in mature glia may also contribute to a decline in CNS health.
These findings dovetail nicely with a recent report from Purice and colleagues (2016), which reveals that translation of the Draper receptor declines significantly with age due to reduced phosphoinositide-3-kinase (PI3K) signaling (33). Consequently, in the senescent brain, glial cells are sluggish responding to axon injury, and glial clearance of degenerating axonal debris is significantly delayed (33). Interestingly, in a Drosophila model of Huntington’s Disease, Draper is also required for glial uptake of neuronally-derived mutant huntingtin (Htt) aggregates (34**). Draper-dependent transfer of mutant Htt aggregates promotes the prion-like pathogenic conversion of soluble wild type Htt peptides within glial cells (34**). Thus, due to a decline in Draper levels, aged glia may be poor at clearing a variety of neurotoxic factors, contributing to a heightened risk for damage and disease. Finally, with regard to proteinopathies, glial cells are viable candidates for transmission of protein aggregates from cell to cell or from one brain region to another, implicating Draper/MEGF10 as a new candidate intervention point to block protein aggregate spreading in specific neurodegenerative disorders.
Aging is associated with impaired PI3K signaling, and the above findings raise the following important question: Which upstream pathways are altered with age to inhibit glial PI3K activity and, subsequently, reduce Draper production? One interesting candidate is the insulin-like signaling pathway, which controls energy homeostasis, cellular growth, and cell survival. In vertebrates, the ILS receptor family includes the insulin receptor and insulin-like growth factor receptors, while the Drosophila genome contains only one related gene, the insulin-like receptor (InR). In all species, this class of receptors initiates a highly conserved canonical cascade that includes PI3K (35). Recent work has revealed a unique role for the insulin-like pathway in the context of glial immune responses to nerve injury in adult flies (36*). Musashe and colleagues (2016) now show that transection of the olfactory nerves in flies triggers increased insulin-like signaling in local ensheathing glia, the glial subtype responsible for phagocytically clearing degenerating axons in the adult brain (36*). Ensheathing glia typically upregulate Draper as they infiltrate injury sites and engulf degenerating olfactory projections (23, 27, 30). Genetic inhibition of the insulin-like pathway in ensheathing glia blocks injury-induced Draper upregulation and delays clearance of severed projections. Importantly, forced expression of Draper in glial-InR depleted flies provides significant rescue with regard to axon engulfment, indicating that the insulin-like pathway as an important upstream positive regulator of Draper and glial phagocytic function (36*). It is well established that systemic insulin-like signaling pathways gradually decline with age across species (37); future work will reveal if this contributes to age-related loss of Draper/MEGF10 and attenuated glial immune responses in older animals. Interestingly, genetic deletion of insulin-like signaling pathway components, including the InR and PI3K, extends lifespan in C. elegans, Drosophila, and mammals, although increased longevity may occur at the cost of optimal metabolic fitness and overall health (38). Selected manipulation of PI3K and insulin-like cascades in a cell type specific and temporal manner may prove to be the most ideal method of optimizing glial immune gene expression and immune-like responses in the aged brain.
Another set of well-characterized immune cascades are the Toll and Imd (immune deficiency) pathways, which both activate conserved Nuclear Factor–κB (NF-κB)-related transcription factors (known as Relish and Dif in flies) to drive expression of immune genes, including those that encode secreted antimicrobial peptides (AMPs). These NF-κB-dependent pathways have been well characterized in the systemic immune system and are essential for destroying invading pathogens and defending overall organismal health (39). We now know that these pathways are also activated in the Drosophila brain and can profoundly influence neuronal survival. For example, glial overexpression of secreted AMPs is sufficient to promote neurodegeneration (40). In fly models of Ataxia-Telangiectasia, activation of the NF-κB homolog Relish in adult glia is also a primary driving force for neurodegeneration (41). Finally, work from Kounatidis and colleagues (2017) also reveals that IMD/NF-κB activity increases as part of normal aging and adversely influences locomotor activity, metabolism, and longevity (42*). Collectively, these studies further emphasize the notion that manipulating glial immunity pathways could serve as a useful strategy to extend health span.
Glia-neuron signaling and implications for neurodegenerative disease
For decades, Drosophila has served as a tractable genetic model to investigate basic molecular mechanisms that underlie neurodegenerative disease pathogenesis (43). The more recent explosion of work related to Drosophila glial cell biology has now poised to us to tackle the role of glia-neuron interactions in the context of disease.
Liu et al. (2015) took a broad approach to investigate how defects common to many neurological disorders influence glial cell function (44**). Using the adult Drosophila retina as a model, they stressed neurons in vivo by genetically inhibiting mitochondrial activity and promoting the production of reactive oxygen species (ROS). Interestingly, these neuronal defects encouraged the formation of lipid droplets (LD) non-cell autonomously in local glial cells, which was followed by neurodegeneration; inhibiting LD formation in glia provided neuroprotection. They went on to show in a mouse model for the neurodegenerative disorder Leigh syndrome that LD formation in astrocytes and microglia preceded neuronal death in various brain regions (44**), suggesting that similar neuron-glia crosstalk mechanisms are conserved in higher organisms. LDs typically serve as storage sites for triglycerides and cholesterol, and the mechanistic significance of LDs in the context of neuronal stress or disease is not yet clear. Nonetheless, because mitochondrial dysfunction and oxidative stress are core components of many neurological disorders, this works offers a new twist on how glia may contribute to disease progression, specifically through altered lipid metabolism.
Drosophila has also furthered our understanding of the complex pathology of Alzheimer’s disease (AD), including molecular, cellular, and behavioral dysfunction. Fly models that express human amyloid precursor protein (APP), amyloid-β42, and/or tau have all provided valuable information about how these neurotoxic peptides influence synaptic signaling and structure, as well as behavioral phenotypes including locomotor defects (45). The fly genome contains one APP-related gene, APP-like (APPL), which, when mutated, causes defects in neuronal outgrowth, synaptic stability, and behavior, and Appl mutant flies have provided important insight into endogenous APP signaling mechanisms in the CNS (46). Across all model organisms, an overwhelming number of studies have explored the role of neuronal APP, but relatively little is known about the function of glial APP, despite the fact that APP is also expressed in mammalian oligodendrocytes, astrocytes, and microglia (21, 47). A recent study explored how glial APPL influences sleep-wake cycles in adult flies. There is a clear reciprocal relationship between Alzheimer’s disease and sleep disruption (48), and this phenomenon extends to Drosophila (49). Farca Luna and colleagues (2017) found that knockdown of APPL in adult fly glia, specifically in cortex glia or astrocytes, alters sleep patterns. Appl mutant flies sleep more overall and display more consolidated sleep during the night, while overexpression of APPL in glia has the opposite effect. Interestingly, boosting glial cells’ ability to uptake glutamate reverses the Appl mutant sleep phenotypes. Notably, glial depletion of APPL also inhibits the expression of a) glutamine synthetase, an enzyme that converts glutamate to glutamine, and b) innexin 2, a component of gap junctions, which allow small molecules to pass between specific glial subtypes (50*). Together, these findings raise the intriguing possibility that disruption of glial APP function or processing promotes intrinsic changes within glial cells to disturb glutamate metabolism and/or recycling, which, in turn, contributes to altered sleep patterns in Alzheimer’s disease patients.
The glial-based leukodystrophy disorder Alexander disease, which presents with developmental delays, loss of myelin, and dementia, is a result of mutations in the gene for glial fibrillary acidic protein (GFAP). Wang and colleagues (2011) developed a robust model for Alexander disease in Drosophila by expressing mutant forms of human GFAP in glia, which recapitulates key features of the disease including abnormal protein folding, dysfunctional glutamate transport, seizures, and neurodegeneration (51). Exploiting the rapid genetic capabilities of the fly, they performed an unbiased forward genetic modifier screen and identified nitric oxide (NO) as a key player in glial-mediated neuronal death induced by GFAP toxicity. After probing candidate upstream and downstream regulators, their work revealed that pathogenic versions of GFAP promote upregulation of the inducible nitric oxide synthase (iNOS) gene via the transcription factor STAT. In turn, excess NO levels non-cell autonomously induce cyclic guanosine monophosphate (cGMP) signaling and apoptosis in neurons (51). An independent in vivo pharmacological screen of FDA-approved drugs identified a cohort of muscarinic acetycholine receptor (mAChR) antagonists that reverse toxicity in Alexander model flies (52). Notably, brain tissue from Alexander model mice and patients also display increased iNOS and mAChR expression in the CNS, although the mechanisms driving the latter change are still unclear (51, 52). Continued screening efforts and histological analysis in flies will likely provide new insight into the mechanisms of this disease and the specific contributions of glial cells.
Conclusion
Our understanding of glial cell biology is still in its infancy, but resolving how glia differentiate, influence brain metabolism, and signal reciprocally with neurons throughout life is essential in order to fully comprehend how the nervous system becomes vulnerable to stress and disease. Here, we briefly describe key recent advances that highlight Drosophila contributions to the fields of normal aging, energetic homeostasis, immunity, and neurodegenerative disease progression. These findings expose novel glia-neuron signaling pathways and further show that invertebrate and vertebrate glia employ common molecular pathways to ensure optimal neuronal function and to defend CNS fitness. Emerging advances in genetic manipulation (e.g. CRISPR/Cas9), high resolution light microscopy, and live microscopy imaging methods promise to strengthen the rich insight we can gain from invertebrates about this utterly important but poorly understood cell type in the brain.
Highlights.
-
-
Drosophila glia are strikingly similar to mammalian counterparts in form and function
-
-
Glial immune responses decline naturally with age
-
-
Innate immune responses influence brain health and disease progression
Acknowledgments
I thank Sean D. Speese for comments on the manuscript. I apologize to colleagues whose primary work I was unable to include due to space constraints. Work in the Logan lab is supported by the National Institutes of Health (NIH) Grant R01 NS079387-01 (M.A.L), NIH Grant R21 NS084112, the Medical Research Foundation of Oregon (M.A.L), the Fred W. Fields Foundation (M.A.L), and the Ken and Ginger Harrison Scholar Award (M.A.L.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
There are no conflicts to declare.
References
- 1.Freeman MR. Drosophila Central Nervous System Glia. Cold Spring Harb Perspect Biol. 2015;7 doi: 10.1101/cshperspect.a020552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schirmeier S, Matzat T, Klämbt C. Axon ensheathment and metabolic supply by glial cells in Drosophila. Brain Res. 2016;1641:122–129. doi: 10.1016/j.brainres.2015.09.003. [DOI] [PubMed] [Google Scholar]
- 3.Coutinho-Budd J, Freeman MR. Probing the enigma: unraveling glial cell biology in invertebrates. Curr Opin Neurobiol. 2013;23:1073–1079. doi: 10.1016/j.conb.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Omoto JJ, Lovick JK, Hartenstein V. Origins of glial cell populations in the insect nervous system. Curr Opin Insect Sci. 2016;18:96–104. doi: 10.1016/j.cois.2016.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Awasaki T, Lai SL, Ito K, Lee T. Organization and postembryonic development of glial cells in the adult central brain of Drosophila. J Neurosci. 2008;28:13742–13753. doi: 10.1523/JNEUROSCI.4844-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Doherty J, Logan MA, Taşdemir OE, Freeman MR. Ensheathing glia function as phagocytes in the adult Drosophila brain. J Neurosci. 2009;29:4768–4781. doi: 10.1523/JNEUROSCI.5951-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Edwards TN, Meinertzhagen IA. The functional organisation of glia in the adult brain of Drosophila and other insects. Prog Neurobiol. 2010;90:471–497. doi: 10.1016/j.pneurobio.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hartenstein V. Morphological diversity and development of glia in Drosophila. Glia. 2011;59:1237–1252. doi: 10.1002/glia.21162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hindle SJ, Bainton RJ. Barrier mechanisms in the Drosophila blood-brain barrier. Front Neurosci. 2014;8:414. doi: 10.3389/fnins.2014.00414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kremer MC, Jung C, Batelli S, Rubin GM, Gaul U. The glia of the adult Drosophila nervous system. Glia. 2017;65:606–638. doi: 10.1002/glia.23115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11*.Charlton-Perkins MA, Sendler ED, Buschbeck EK, Cook TA. Multifunctional glial support by Semper cells in the Drosophila retina. PLoS Genet. 2017;13:e1006782. doi: 10.1371/journal.pgen.1006782. Here the authors describe the molecular, structural, and functional signature of glial Semper cells, which function similarly to mammalian Mueller glia in regulating photoreceptor activity in the adult retina. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DeSalvo MK, Hindle SJ, Rusan ZM, Orng S, Eddison M, Halliwill K, Bainton RJ. The Drosophila surface glia transcriptome: evolutionary conserved blood-brain barrier processes. Front Neurosci. 2014;8:346. doi: 10.3389/fnins.2014.00346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang Y, Ng FS, Jackson FR. Comparison of larval and adult Drosophila astrocytes reveals stage-specific gene expression profiles. G3 (Bethesda) 2015;5:551–558. doi: 10.1534/g3.114.016162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ng FS, Jackson FR. The ROP vesicle release factor is required in adult Drosophila glia for normal circadian behavior. Front Cell Neurosci. 2015;9:256. doi: 10.3389/fncel.2015.00256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bolaños JP. Bioenergetics and redox adaptations of astrocytes to neuronal activity. J Neurochem. 2016;139(Suppl 2):115–125. doi: 10.1111/jnc.13486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fünfschilling U, Supplie LM, Mahad D, Boretius S, Saab AS, Edgar J, Brinkmann BG, Kassmann CM, Tzvetanova ID, Möbius W, Diaz F, Meijer D, Suter U, Hamprecht B, Sereda MW, Moraes CT, Frahm J, Goebbels S, Nave KA. Glycolytic oligodendrocytes maintain myelin and long-term axonal integrity. Nature. 2012;485:517–521. doi: 10.1038/nature11007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee Y, Morrison BM, Li Y, Lengacher S, Farah MH, Hoffman PN, Liu Y, Tsingalia A, Jin L, Zhang PW, Pellerin L, Magistretti PJ, Rothstein JD. Oligodendroglia metabolically support axons and contribute to neurodegeneration. Nature. 2012;487:443–448. doi: 10.1038/nature11314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Verkhratsky A, Nedergaard M. Astroglial cradle in the life of the synapse. Philos Trans R Soc Lond B Biol Sci. 2014;369:20130595. doi: 10.1098/rstb.2013.0595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Volkenhoff A, Weiler A, Letzel M, Stehling M, Klämbt C, Schirmeier S. Glial Glycolysis Is Essential for Neuronal Survival in Drosophila. Cell Metab. 2015;22:437–447. doi: 10.1016/j.cmet.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 20.Miller D, Hannon C, Ganetzky B. A mutation in Drosophila Aldolase causes temperature-sensitive paralysis, shortened lifespan, and neurodegeneration. J Neurogenet. 2012;26:317–327. doi: 10.3109/01677063.2012.706346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cahoy JD, Emery B, Kaushal A, Foo LC, Zamanian JL, Christopherson KS, Xing Y, Lubischer JL, Krieg PA, Krupenko SA, Thompson WJ, Barres BA. A transcriptome database for astrocytes, neurons, and oligodendrocytes: a new resource for understanding brain development and function. J Neurosci. 2008;28:264–278. doi: 10.1523/JNEUROSCI.4178-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chung WS, Clarke LE, Wang GX, Stafford BK, Sher A, Chakraborty C, Joung J, Foo LC, Thompson A, Chen C, Smith SJ, Barres BA. Astrocytes mediate synapse elimination through MEGF10 and MERTK pathways. Nature. 2013;504:394–400. doi: 10.1038/nature12776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.MacDonald JM, Beach MG, Porpiglia E, Sheehan AE, Watts RJ, Freeman MR. The Drosophila cell corpse engulfment receptor Draper mediates glial clearance of severed axons. Neuron. 2006;50:869–881. doi: 10.1016/j.neuron.2006.04.028. [DOI] [PubMed] [Google Scholar]
- 24.Scheib JL, Sullivan CS, Carter BD. Jedi-1 and MEGF10 signal engulfment of apoptotic neurons through the tyrosine kinase Syk. J Neurosci. 2012;32:13022–13031. doi: 10.1523/JNEUROSCI.6350-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tasdemir-Yilmaz OE, Freeman MR. Astrocytes engage unique molecular programs to engulf pruned neuronal debris from distinct subsets of neurons. Genes Dev. 2014;28:20–33. doi: 10.1101/gad.229518.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu HH, Bellmunt E, Scheib JL, Venegas V, Burkert C, Reichardt LF, Zhou Z, Farinas I, Carter BD. Glial precursors clear sensory neuron corpses during development via Jedi-1, an engulfment receptor. Nat Neurosci. 2009;12:1534–1541. doi: 10.1038/nn.2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Doherty J, Sheehan AE, Bradshaw R, Fox AN, Lu TY, Freeman MR. PI3K signaling and Stat92E converge to modulate glial responsiveness to axonal injury. PLoS Biol. 2014;12:e1001985. doi: 10.1371/journal.pbio.1001985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lu TY, MacDonald JM, Neukomm LJ, Sheehan AE, Bradshaw R, Logan MA, Freeman MR. Axon degeneration induces glial responses through Draper-TRAF4-JNK signalling. Nat Commun. 2017;8:14355. doi: 10.1038/ncomms14355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Macdonald JM, Doherty J, Hackett R, Freeman MR. The c-Jun kinase signaling cascade promotes glial engulfment activity through activation of draper and phagocytic function. Cell Death Differ. 2013;20:1140–1148. doi: 10.1038/cdd.2013.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Logan MA, Hackett R, Doherty J, Sheehan A, Speese SD, Freeman MR. Negative regulation of glial engulfment activity by Draper terminates glial responses to axon injury. Nat Neurosci. 2012;15:722–730. doi: 10.1038/nn.3066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Draper I, Mahoney LJ, Mitsuhashi S, Pacak CA, Salomon RN, Kang PB. Silencing of drpr leads to muscle and brain degeneration in adult Drosophila. Am J Pathol. 2014;184:2653–2661. doi: 10.1016/j.ajpath.2014.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Etchegaray JI, Elguero EJ, Tran JA, Sinatra V, Feany MB, McCall K. Defective Phagocytic Corpse Processing Results in Neurodegeneration and Can Be Rescued by TORC1 Activation. J Neurosci. 2016;36:3170–3183. doi: 10.1523/JNEUROSCI.1912-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Purice MD, Speese SD, Logan MA. Delayed glial clearance of degenerating axons in aged Drosophila is due to reduced PI3K/Draper activity. Nat Commun. 2016;7:12871. doi: 10.1038/ncomms12871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34**.Pearce MM, Spartz EJ, Hong W, Luo L, Kopito RR. Prion-like transmission of neuronal huntingtin aggregates to phagocytic glia in the Drosophila brain. Nat Commun. 2015;6:6768. doi: 10.1038/ncomms7768. Mutant huntingtin aggregates are handed off to local phagocytic glia, promoting prion-like conversion of wild type huntingtin in glial cells. The authors show that mechanism requires the conserved Draper/MEGF10 engulfment receptor. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Broughton S, Partridge L. Insulin/IGF-like signalling, the central nervous system and aging. Biochem J. 2009;418:1–12. doi: 10.1042/BJ20082102. [DOI] [PubMed] [Google Scholar]
- 36*.Musashe DT, Purice MD, Speese SD, Doherty J, Logan MA. Insulin-like Signaling Promotes Glial Phagocytic Clearance of Degenerating Axons through Regulation of Draper. Cell Rep. 2016;16:1838–1850. doi: 10.1016/j.celrep.2016.07.022. The authors define a novel role for the conserved insulin-like signaling pathways in directing glial phagocytic responses to nerve injury through regulation of innate gene expression. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Piriz J, Muller A, Trejo JL, Torres-Aleman I. IGF-I and the aging mammalian brain. Exp Gerontol. 2011;46:96–99. doi: 10.1016/j.exger.2010.08.022. [DOI] [PubMed] [Google Scholar]
- 38.Riera CE, Merkwirth C, De Magalhaes Filho CD, Dillin A. Signaling Networks Determining Life Span. Annu Rev Biochem. 2016;85:35–64. doi: 10.1146/annurev-biochem-060815-014451. [DOI] [PubMed] [Google Scholar]
- 39.Kingsolver MB, Huang Z, Hardy RW. Insect antiviral innate immunity: pathways, effectors, and connections. J Mol Biol. 2013;425:4921–4936. doi: 10.1016/j.jmb.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cao Y, Chtarbanova S, Petersen AJ, Ganetzky B. Dnr1 mutations cause neurodegeneration in Drosophila by activating the innate immune response in the brain. Proc Natl Acad Sci U S A. 2013;110:E1752–60. doi: 10.1073/pnas.1306220110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Petersen AJ, Katzenberger RJ, Wassarman DA. The innate immune response transcription factor relish is necessary for neurodegeneration in a Drosophila model of ataxia-telangiectasia. Genetics. 2013;194:133–142. doi: 10.1534/genetics.113.150854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42*.Kounatidis I, Chtarbanova S, Cao Y, Hayne M, Jayanth D, Ganetzky B, Ligoxygakis P. NF-κB Immunity in the Brain Determines Fly Lifespan in Healthy Aging and Age-Related Neurodegeneration. Cell Rep. 2017;19:836–848. doi: 10.1016/j.celrep.2017.04.007. This manuscript investigates the complex interplay between innate glial immune pathways, longevity, and health span in Drosophila and shows that the evolutionary conserved NF-κB immune pathway contributes to both healthy aging and neurodegenerative disease progression. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McGurk L, Berson A, Bonini NM. Drosophila as an In Vivo Model for Human Neurodegenerative Disease. Genetics. 2015;201:377–402. doi: 10.1534/genetics.115.179457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44**.Liu L, Zhang K, Sandoval H, Yamamoto S, Jaiswal M, Sanz E, Li Z, Hui J, Graham BH, Quintana A, Bellen HJ. Glial lipid droplets and ROS induced by mitochondrial defects promote neurodegeneration. Cell. 2015;160:177–190. doi: 10.1016/j.cell.2014.12.019. Neuronal stress triggers lipid droplet (LD) formation in glial cells. Pharmacological or genetic inhibition of glial LDs delays neurodegeneration, indicating that LDs may serve as useful biomarkers and intervention points in the context of disease. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Prüßing K, Voigt A, Schulz JB. Drosophila melanogaster as a model organism for Alzheimer’s disease. Mol Neurodegener. 2013;8:35. doi: 10.1186/1750-1326-8-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cassar M, Kretzschmar D. Analysis of Amyloid Precursor Protein Function in Drosophila melanogaster. Front Mol Neurosci. 2016;9:61. doi: 10.3389/fnmol.2016.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Oberstein TJ, Spitzer P, Klafki HW, Linning P, Neff F, Knölker HJ, Lewczuk P, Wiltfang J, Kornhuber J, Maler JM. Astrocytes and microglia but not neurons preferentially generate N-terminally truncated Aβ peptides. Neurobiol Dis. 2015;73:24–35. doi: 10.1016/j.nbd.2014.08.031. [DOI] [PubMed] [Google Scholar]
- 48.Ju YE, Lucey BP, Holtzman DM. Sleep and Alzheimer disease pathology–a bidirectional relationship. Nat Rev Neurol. 2014;10:115–119. doi: 10.1038/nrneurol.2013.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tabuchi M, Lone SR, Liu S, Liu Q, Zhang J, Spira AP, Wu MN. Sleep interacts with aβ to modulate intrinsic neuronal excitability. Curr Biol. 2015;25:702–712. doi: 10.1016/j.cub.2015.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50*.Farca Luna AJ, Perier M, Seugnet L. Amyloid Precursor Protein in Drosophila Glia Regulates Sleep and Genes Involved in Glutamate Recycling. J Neurosci. 2017;37:4289–4300. doi: 10.1523/JNEUROSCI.2826-16.2017. Glial App influences sleep/wake patterns in adult animals and disruption of glial App perturbs these normal patterns by disrupting glutamate recycling in the brain. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang L, Hagemann TL, Kalwa H, Michel T, Messing A, Feany MB. Nitric oxide mediates glial-induced neurodegeneration in Alexander disease. Nat Commun. 2015;6:8966. doi: 10.1038/ncomms9966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang L, Hagemann TL, Messing A, Feany MB. An In Vivo Pharmacological Screen Identifies Cholinergic Signaling as a Therapeutic Target in Glial-Based Nervous System Disease. J Neurosci. 2016;36:1445–1455. doi: 10.1523/JNEUROSCI.0256-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]


