Abstract
Recent research indicates the relative benefits of computerized attention control treatment (ACT) and attention bias modification treatment (ABMT) for posttraumatic stress disorder (PTSD); however, neural changes underlying these therapeutic effects remain unknown. This study examines how these two types of attention training modulate neurological dysfunction in veterans with PTSD. A community sample of 46 combat veterans with PTSD participated in a randomized double-blinded clinical trial of ACT versus ABMT and 32 of those veterans also agreed to undergo resting-state magnetoencephalography (MEG) recordings. Twenty-four veterans completed psychological and MEG assessments at pre- and post-training to evaluate treatment effects. MEG data were imaged using an advanced Bayesian reconstruction method and examined using statistical parametric mapping. In this report, we focus on the neural correlates and the differential treatment effects observed using MEG; the results of the full clinical trial have been described elsewhere. Our results indicated that ACT modulated occipital and ABMT modulated medial temporal activity more strongly than the comparative treatment. PTSD symptoms decreased significantly from pre- to post-test. These initial neurophysiological outcome data suggest that ACT modulates visual pathways, while ABMT modulates threat-processing regions, but that both are associated with normalizing aberrant neural activity in veterans with PTSD.
Keywords: magnetoencephalography, MEG, cortical, PTSD, treatment, attention training
1. Introduction
Posttraumatic stress disorder (PTSD) is a serious psychiatric diagnosis marked by re-experiencing, avoidance, mood, cognitive, and hyperarousal symptoms (American Psychiatric Association, 2013), which affects about a quarter of U.S. veterans serving in recent conflicts in Iraq and Afghanistan (Bagalman, 2013). Approaches such as cognitive behavioral therapy (e.g. prolonged exposure therapy) and pharmacotherapy (e.g. SSRIs) are known to reduce symptoms of PTSD (Forbes et al., 2010), but technology-based and assisted interventions have been recently promoted as ways to improve treatment access for patients with PTSD (Foa et al., 2013; Olthuis et al., 2016; Rosen et al., 2017). Further, no existing treatments are fully effective for all patients with PTSD, and thus new treatments that target perturbed brain function in PTSD are direly needed.
Attention Bias Modification Treatment (ABMT) is an emerging therapy for anxiety disorders, which involves training attention away from threatening stimuli using a dot-probe task (Bar-Haim, 2010). Meta-analyses suggest that ABMT reduces vigilance toward threat-related attention biases (Hakamata et al., 2010; Hallion and Ruscio, 2011) in various samples, including in patients with social phobia (Schmidt et al., 2009) and generalized anxiety disorder (Amir et al., 2009). In comparison, PTSD presents a more complicated picture with studies indicating either threat-related avoidance (Buckley et al., 2000; Fani et al., 2012) or vigilance (Beevers et al., 2011; Constans et al., 2004; Bar-Haim et al., 2010; Wald et al., 2011), as well as heightened moment-to-moment fluctuations in attention bias, termed “attention-bias variability” (Iacoviello et al., 2014; Naim et al., 2015). We recently demonstrated that attention control training (ACT), which trains participants to disregard irrelevant threat-related contingencies and focus instead on the completing the visual dot-probe task, is more effective than ABMT in reducing PTSD symptoms, possibly by normalizing attention bias variability (Badura-Brack et al., 2015).
While previous research indicates the relative benefits of ACT and ABMT for PTSD (Badura-Brack et al., 2015; Kuckertz et al., 2014; Schoorl et al., 2013), the neural changes underlying these therapeutic effects remain unknown. Early imaging studies comparing patients with PTSD to healthy controls have identified functional abnormalities in the ventromedial prefrontal cortex, anterior cingulate, hippocampi, amygdalae, and insula (Morey et al., 2012; Pitman et al., 2012; Rabinak et al., 2011; Sripada et al., 2012). More recently, studies have reported abnormalities in broad-based cortical areas including the parietal, prefrontal, motor, and occipital areas, as well as the default-mode network in PTSD (Badura-Brack, et al., 2017; Gong et al., 2014; Eckart et al., 2011; Schuff et al., 2011; Liu et al., 2012; Mueller-Pfeiffer et al., 2012), and fMRI studies have reported altered resting-state functional connectivity in participants with PTSD compared to non-clinical samples (Lanius et al., 2009; Qin et al., 2012; Zhou et al., 2012). Several resting-state magnetoencephalography (MEG) studies have also reported hyper-connectivity in medial temporal regions in PTSD (Dunkley et al., 2015, 2014), high-frequency hyper-synchrony especially in the left temporal sub-network (Misic, et al.,2016), and distinct patterns of neural activity during resting state as compared to healthy participants (Huang, et al., 2014). Such findings correspond with cognitive models of PTSD implicating altered attention patterns and perturbed perceptual processes,which have been linked theoretically (Ehlers and Clark, 2000) and empirically to PTSD (Todd et al., Dunkley, et al. 2015, Khanna, et al. (in press), McDermott et al., 2016). Thus, the disorder appears to involve dysfunction across multiple brain regions and cognitive functions, including regions not typically associated with fear or threat detection.
In the current study, we used MEG to assess whether ACT and ABMT differentially affected spontaneous resting-state neural activity in patients with PTSD. In a recent non-therapeutic study that used MEG and a virtually identical data processing approach to that used in the current study, we found that veterans with PTSD exhibited stronger spontaneous neural activity in prefrontal, sensorimotor, temporal, and medial temporal regions and weaker occipital activity than exhibited by combat veterans without PTSD (Badura-Brack et al., 2017). Given these findings and those of our clinical trial (Badura-Brack et al., 2017, 2015), we hypothesized that ACT and ABMT would differentially modulate brain activity in specific areas in veterans with PTSD. In this exploratory MEG study, our preliminary hypotheses were that treatments would reduce spontaneous activity in threat processing areas in the medial temporal lobe that are commonly hyperactive in PTSD, as well as modulate attention and visual processing brain areas due to the visual nature of the attention training interventions.
2. Methods
Participants were selected from a larger sample of 46 male combat veterans with PTSD who served in a warzone in Iraq or Afghanistan between 2003-2014. These veterans took part in a clinical trial (NCT01564667) with complete methods and results reported elsewhere (BaduraBrack et al., 2015). A total of 32 of those patients with PTSD also consented and qualified to complete MEG recordings. The current study reports results from the 24 veterans (12 receiving ACT and 12 receiving ABMT) who completed the clinical trial and both pre and post-intervention MEG recordings and psychological assessments. See Figure 1 for a diagram of participant flow through the study. As a validity check, we examined scores for those veterans who completed treatment versus those who dropped out of treatment, and the participants did not vary significantly on the key variables of lifetime trauma exposure, combat trauma exposure, or PTSD severity.
Figure 1.
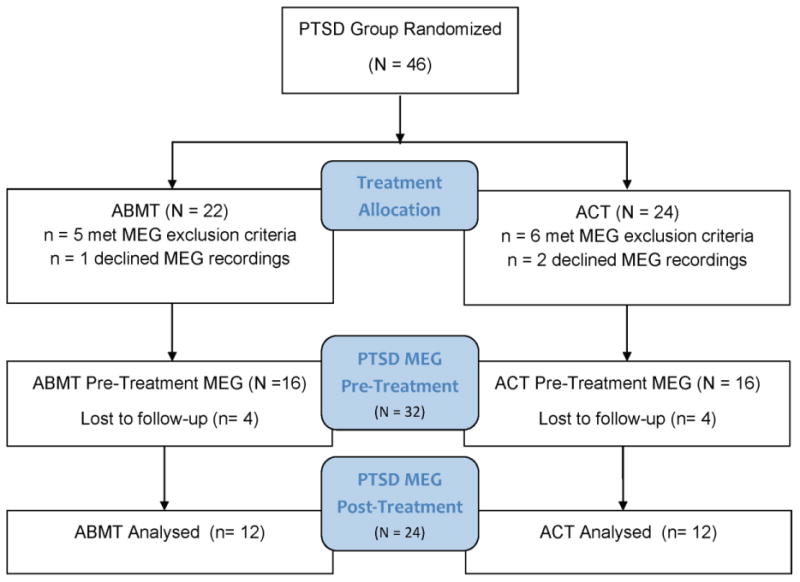
CONSORT diagram for participant flow through treatment allocation and participation in pre- and post- treatment MEG recordings.
PTSD was diagnosed using the Clinician Administered PTSD Scale (CAPS) (Blake et al., 1995) using the CAPS F1/I2 rule (Weathers et al., 1999). The Mini International Neuropsychiatric Interview (M.I.N.I.) (Sheehan et al., 1998) was used to exclude participants with comorbid disorders including psychosis, bipolar disorder, obsessive-compulsive disorder, or current substance dependence; however veterans with depressive and anxious symptoms were allowed to participate due to the strong link between these diagnoses and PTSD. All participants also completed the Patient Health Questionnaire (PHQ-9) to assess depression (Kroenke et al., 2001); the Toronto Alexithymia Scale (TAS-20) to measure difficulty identifying and describing feelings (Bagby et al., 1994); the State-Trait Anxiety Inventory (STAI; Spielberger et al., 1983); the Deployment Risk and Resilience Inventory (DRRI) combat exposure scale (Vogt et al., 2008); and the Life Events Checklist (LEC) to quantify traumatic events across the lifespan (Blake et al. 1995). Veterans were excluded from the trial if they were receiving current psychotherapy; however, they were allowed to continue on their psychotropic medications, as long as they had been on a steady dosage for at least six months prior to beginning the study and did not change medications or doses during the trial. With regard to medications, 17% of the total sample were on a steady dose of an SSRI, and 25% of the total sample were on a steady dose of a mood stabilizer. Six participants in the ACT group were taking steady doses of psychotropic medication (3 mood stabilizer, 3 SSRI) as compared to four (3 mood stabilizer, 1 SSRI) in the ABMT group. General exclusionary criteria included any medical diagnosis affecting CNS function, known brain neoplasm or lesion, history of significant head trauma, current substance dependence, and ferromagnetic implants. For enrollment information and flow through the clinical trial and MEG recordings, see Figure 1. Written informed consent was obtained following the guidelines of the Institutional Review Board of Creighton University, who approved the study protocol.
2.1. Attention bias treatment (ACT and ABMT)
For complete details on the methods and results of the treatment trial, see (Badura-Brack et al., 2015). In brief, this double-blinded study randomly assigned participants with PTSD to one of two attention-training conditions: ACT or ABMT. In both conditions, pairs of faces, one angry and one neutral, were presented one above the other (simultaneously) on a computer screen and immediately followed by a visual probe appearing in the location vacated by one of the faces. Participants were required to detect probe type (< or >) as quickly as they could by pressing the left or right button on a computer mouse. In ACT, the probe appeared equally at the angry and neutral faces locations, balancing fluctuations in attention toward and away from threat and training participants to ignore irrelevant threat information and focus on the task at hand. In ABMT, the probe appeared in the space vacated by the neutral face, implicitly teaching participants to focus attention away from threat. Participants completed 8 training sessions at a rate of two 10-15 minute sessions per week for a total of four weeks.
2.2. MEG methods: Data acquisition & analyses
Participants were seated with both arms resting on a tray attached to the chair body inside the MEG chamber. They were instructed to relax, remain still, and to stay awake with their eyes closed for one continuous 6-minute recording. They repeated the same MEG recording paradigm after completing the eight sessions of ACT or ABMT.
Neuromagnetic responses were sampled continuously at 1 kHz using an Elekta MEG system with 306 sensors. MEG data were corrected for head motion, subjected to advanced noise reduction (Taulu and Simola, 2006), coregistered to structural MRI data, and divided into epochs of 4096 ms duration. Epochs with artifacts were identified using an automated searching procedure that marked flat and high amplitude segments for rejection, supplemented with visual inspection. Artifact-free epochs were filtered 1-54 Hz and downsampled to 180 Hz prior to image reconstruction to reduce computational burden. The downsampled data were then imaged using the Bayesian multiple sparse priors (MSP) approach implemented in the Statistical Parametric Mapping software (SPM12; Wellcome Trust Centre for Neuroimaging). This approach involves estimating the source priors for reconstruction in the Bayesian framework.
In our MSP approach, source space consisted of 8196 dipolar voxels equally-distributed throughout gray matter. Data covariance was calculated across the whole epoch from 1-54 Hz. Prior to inversion, the MEG sensor data were transformed into a set of orthogonal modes using a singular value decomposition over the lead field matrix, and a temporal projector was applied to reduce the data to 16 temporal modes (Friston et al., 2008; Henson et al., 2009; López et al., 2013). A Variational Laplace approach was then used to estimate the combination of hyperparameters that maximized free energy in the Bayesian framework. Model selection used both automatic relevance determination and greedy search schemes, and the covariance matrices produced by each and the sensor noise covariances were mixed using Variational Laplace in a second inversion step. The resulting single covariance matrix was used to get the posterior mean and variance of the current density, with output images written at 2 × 2 × 2 mm resolution. See Lopez et al .(2013) for a detailed description of the MSP approach to Bayesian source reconstruction.
The resulting 3D maps of functional brain activity were statistically evaluated in SPM12 using a mass univariate approach based on the general linear model. Briefly, paired-sample t-tests were conducted to probe treatment effects in each group, and two-sample t-tests were applied to the treatment difference images (pre-treatment – post-treatment) to probe differential treatment effects. All statistical maps were thresholded at p < 0.01 and for multiple comparisons correction using a spatial extent threshold (cluster restriction), which was calculated directly from the data according to the theory of Gaussian random fields. All statistical analyses for behavioral and clinical variables were conducted in SPSS (Release 21.0.0).
3. Results
3.1. Participant Demographics & Clinical Measures
The mean age of the ACT group was 32.33 (SD: 7.08), and the mean age of the ABMT group was 33.25 (SD: 5.48). The mean educational level of the ACT group was 15.08 (SD: 2.97), and the mean educational level of the ABMT group was 15.17 (SD: 2.48). Veterans in the ACT and ABMT groups did not statistically differ on either measure. Furthermore, prior to treatment, the ACT and ABMT groups did not differ on PTSD severity, or on other variables typically associated with PTSD including depression, state anxiety, trait anxiety, combat exposure, or lifetime trauma exposure. The ACT group did score significantly higher than the ABMT group on alexithymia (difficulty identifying and describing emotions) at pre-test; however this finding did not survive Bonferroni correction (See Table 1). Those taking psychotropic medications had more severe PTSD (M = 78.70, SD = 11.18) at intake than those not taking medications (M = 64.50, SD = 15.74), but no medication by treatment effects were noted. A repeated-measures ANOVA on CAPS scores measuring PTSD severity indicated a significant within subjects main effect for time (pre- to post-treatment), F22,1 = 33.26 (p < 0.001), but no main effect of group (ACT, ABMT) or interaction effect. Note that this result differed from the full clinical trial (Badura-Brack et al., 2015), which involved a larger sample and thus increased statistical power. As per the main effect of time, the ACT group had a mean pre-treatment CAPS of 73.67 (SD: 16.08) and post-treatment CAPS of 47.17 (SD: 21.89), while the ABMT group had a mean pre-treatment CAPS was 67.17 (SD: 14.84), and post-treatment CAPS of 47.08 (SD: 19.09). Thus, symptom severity significantly decreased in both groups from pre- to post-treatment.
Table 1. Psychological Symptom Measures at Intake by Treatment Group.
| Measure (symptom) | Group | Mean (SD) |
|---|---|---|
| PHQ-9 (depression) | ACT | 14.50 (6.05) |
| ABMT | 10.17 (4.76) | |
| TAS-20 (alexithymia) * | ACT | 60.58 (9.95) |
| ABMT | 49.92 (13.71) | |
| STAI Y1 (state anxiety) | ACT | 46.17 (7.74) |
| ABMT | 42.33 (9.50) | |
| STAI Y2 (trait anxiety) | ACT | 50.75 (9.52) |
| ABMT | 43.08 (13.40) | |
| DRRI (combat exposure) | ACT | 39.83 (19.61) |
| ABMT | 37.67 (16.52) | |
| Life Events Checklist (trauma exposure) | ACT | 39.67 (12.40) |
| ABMT | 43.83 (11.64) |
ACT (n = 12); ABMT (n = 12)
3.2. Neurophysiological ACT and ABMT treatment effects
Given the aims of the study, we first conducted paired-samples t-tests on the ACT and ABMT groups separately to identify how each modulated resting-state brain activity. We then followed this up with two-sample t-tests to identify brain regions that were more strongly modulated by one therapy relative to the other. Peak coordinates for each region are noted in context below. Comparing pre- to post-training, the ACT group showed significantly decreased spontaneous neural activity (1-54 Hz) in the left (MNI Coordinates: -16, -96, 10) and right (34, -89, 2) posterior occipital cortices, along with increased spontaneous activity in the right lateral occipital cortices (48, -68, 12) after ACT (p < 0.01, corrected). The ACT group also exhibited decreased activity in the left lateral parahippocampal area (-33, -22, -24) following therapy (p < 0.01, corrected; Figure 2). In contrast, the ABMT group showed decreased spontaneous activity in the left precentral and postcentral gyri (-44, -16, 46 and -34, -38, 62) and bilateral medial temporal structures (35, -20, -23 and -26, -18, -28), including the parahippocampal gyri, hippocampi, and the amygdalae from pre- to post-treatment (p < 0.01, corrected; Figure 2). The ABMT group also had increased activity in a region of the left posterior occipital cortices (-22, -94, 6) after treatment (p < 0.01, corrected). In regard to differential treatment effects, we first compared the baseline MEG data between treatment types, which importantly revealed no differences prior to treatment. Next, we examined differential treatment effects (see methods) and found that ACT more strongly affected spontaneous activity in occipital regions, especially left occipital cortex (-22, -98, 5), compared to ABMT. Conversely, ABMT modulated neural activity in bilateral medial temporal structures (i.e., parahippocampal/ hippocampal and amydgala; 32, -1, -28 and -26, -3, -29) more strongly than ACT (p < 0.01, corrected; Figure 2).
Figure 2.
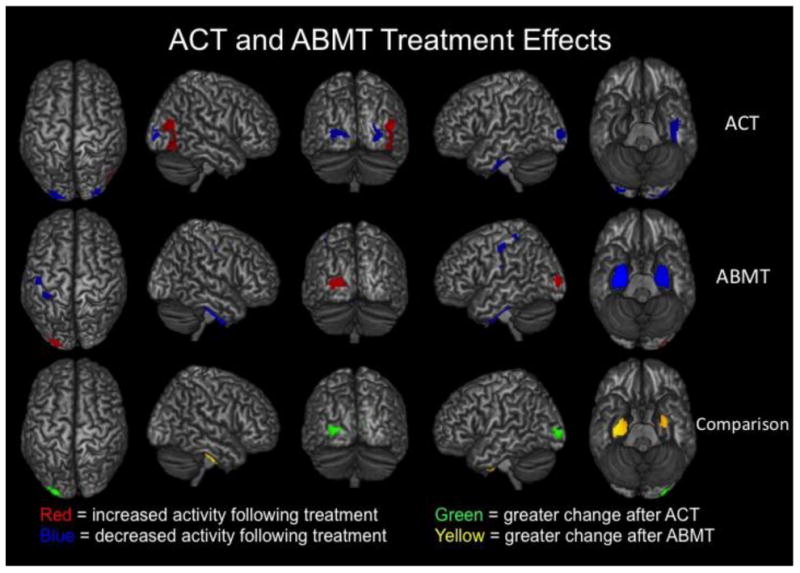
Both ACT and ABMT reduced PTSD symptoms and modulated neuronal activity during the resting-state, but the two therapies affected largely distinct brain regions. In the top two rows, brain regions that exhibited significantly (p < 0.01, corrected) increased activity following therapy are shown in red, whereas those with significantly (p <0 .01, corrected) decreased activity following therapy are shown in blue. Results for pre- to post-ACT appear in the top row and those for pre- to post- ABMT are directly below in the middle row. As shown, ACT tended to modulate posterior and lateral occipital regions, as well as left medial temporal areas (p <0.01, corrected). In contrast, ABMT affected sensorimotor, left posterior occipital, and a large volume of tissue in bilateral medial temporal regions including the amygdalae and parahippocampal complex (p <0 .01, corrected). The two therapies also exerted significantly different effects in some brain regions, and these are shown in the bottom row. First, differential images were created for each treatment (pre-treatment – post-treatment) and the a two-sample t-test was conducted. Note that that pretreatment differences were probed between groups and none were detected. This two-sample t-test showed that ABMT decreased neuronal activity in bilateral medial temporal regions significantly more than ACT, while ACT modulated occipital activity significantly more strongly than ABMT (p < 0.01, corrected).
4. Discussion
We used high-density MEG and advanced image reconstruction methods to identify how ACT and ABMT may modulate resting-state neuronal activity in veterans with PTSD. We found that both types of therapy modulated occipital cortices and medial temporal structures, which is interesting given our recent findings that these brain circuits are abnormal in a sample veterans with PTSD, which included all the veterans in this current study, as compared to a matched sample healthy combat-exposed veterans (Badura-Brack et al., 2017). Specifically, our recently published resting-state non-therapeutic study found that veterans with PTSD had abnormal neural activity in occipital, medial temporal, sensorimotor, and other brain areas relative to veterans without PTSD. Thus, both ABMT and ACT modulated many of the brain regions that were previously identified as abnormal in these veterans with PTSD as compared to psychologically healthy, combat exposed veterans.
In a previous clinical trial, we found that both types of attention training reliably reduce PTSD symptoms, but that ACT was significantly more efficacious for PTSD than ABMT (Badura-Brack et al., 2015). ACT appears to work through normalization of attention bias variability, as opposed to the proposed mechanism of action in ABMT, which focuses only on reducing attention toward threat. Some studies have characterized PTSD as driven by increased attunement to potential threat (e.g., Todd et al., 2015) and driven by fear-based neural mechanisms (for a review, see Pitman, et al., 2012); however, PTSD is also characterized by difficulties in attentional control and executive function (e.g., Leskin & White, 2007; Aupperle et al., 2012; Blair et al., 2013) consistent with an intrusion model of symptoms (e.g. Brewin and colleagues 2014, 2010). Because people with PTSD fluctuate between symptoms of over-attending to threat and avoiding threat, PTSD is better characterized by jointly considering the involvement of both executive dysfunction and enhanced threat detection mechanisms (e.g., Khanna, et al., 2016; Cisler et al., 2011; Pannu Hayes et al., 2009), consistent with the observation of higher levels of attention bias variability in PTSD (Naim et al. 2015, Iacoviello et al., 2014). Our MEG results bolster this combined view, and demonstrate that different attention training approaches (both associated with reductions in PTSD symptoms) modulate different brain regions in PTSD.
Although PTSD symptom severity decreased over time, we should note that differential PTSD treatment effects were not observed in this smaller sample, and this is a limitation of the current study. However, it should also be noted that the ACT group improved by seven more points on the CAPS than the ABMT group, consistent with findings from the full clinical trial. In this study's direct ACT and ABMT comparisons of MEG data, ACT produced significantly greater reductions in left posterior occipital activity as compared to ABMT, and ABMT resulted in significantly greater decreases in activity in the bilateral regions of the parahippocampal complex compared with ACT. Importantly these observed differential effects were in the direction of the healthy controls in the group differences resting state study (Badura-Brack et al., 2017), and impact regions of demonstrated importance in PTSD (Gong et al., 2014; Liu et al., 2012; Morey et al., 2012; Mueller-Pfeiffer et al., 2012; Pitman et al., 2012; Sripada et al., 2012; Rabinak et al., 2011; Eckart et al., 2011; Schuff et al., 2011).
Although we are reporting the results of an exploratory study here, these current findings shed light on potential mechanisms driving PTSD symptom improvement subsequent to ACT and ABMT. Regarding the efficacy of ACT, influential theories in PTSD suggest that re-experiencing symptoms derive from visual sensory impressions inadequately integrated into autobiographical memory at the time of the trauma and reflect disruptions in cognitive processing (Brewin et al., 2010, 1996; Ehlers and Clark, 2000). Because involuntary memory intrusions in PTSD tend to have visual details predominating (Ehlers et al., 2002; Hackmann et al., 2004), some posttraumatic symptoms may be related to priming of these visual memories. Brewin and Burgess (2014) suggest that PTSD symptoms may be ameliorated by either enhancing ventral stream processing (typical of most PTSD psychotherapies) or interfering with dorsal stream processing, which may be the mechanism of action behind ACT, as engaging in visuospatial tasks during or shortly after trauma exposure blocks the development of involuntary images (Brewin, 2014). In ACT, participants see threatening faces risking priming involuntary traumatic intrusions; however, participants are immediately thereafter required to engage in another visuospatial task (identifying the direction of the probe), which may limit processing of these images. Perhaps the reduction in attention bias variability associated with ACT (BaduraBrack et al., 2015) is evidence of a reduced tendency to fluctuate between over- and under-attending to threat in response to involuntary intrusions. This normalization may include increased lateral occipital activity and reduced posterior occipital responses in PTSD patients treated with ACT, as observed in the current study.
Conversely, ABMT encourages threat avoidance directed at reducing fear responses, which should involve alterations in medial temporal lobe activity. The amygdala, hippocampus, and surrounding structures are of known importance in PTSD (Morey et al., 2012; Pitman et al., 2012; Rabinak et al., 2011; Sripada et al., 2012), and the significant changes in these areas in the current study logically follow the symptom improvement as measured by pre- and post-intervention clinical interviews. These parahippocampal regions are also critical memory structures along the visual processing stream and are relevant to how traumatic information is stored and processed in PTSD (Brewin and Burgess, 2014; Ehlers et al., 2002). Because ABMT is designed to train participants to attend away from threat; paraphippocampal brain areas associated with threat detection should be affected by ABMT and this is consistent with our findings in the current study.
Future studies should build on these preliminary findings, as well as address limitations inherent in the current study by including women and survivors of various traumas, and increasing sample sizes in each treatment condition. This sample was not medication free (42% of participants were taking a psychotropic medication), but they did serve as their own controls because they were on steady dose of medication for at least 6 months prior to entering the study and they continued on that steady dose throughout the study. Those veterans who were taking psychotropic medications reported more severe levels of PTSD both before and after training, but their symptoms improved with both versions of attention training. A methodological limitation of this study is that we examined only the resting state using MEG, and some of our findings in deeper structures will need to be confirmed by future studies; however, MEG studies reporting neural activity in deeper brain structures are becoming more common (e.g. Cornwell et al. 2010, 2012, 2014; Dalal et al. 2008; Kessler et al. 2006; McDermott et al. 2016; Muthuraman et al. 2014; Proskovec et al. 2016; Pu et al. 2017; Salvadore et al. 2009, 2010; Wilson et al. 2009, 2010, 2011, 2017). In this study we used both magnetometers and gradiometers for source reconstruction, and magnetometers are inherently more sensitive to neural activity in distant brain areas. We also observed treatment differences in these deeper structures (e.g., amygdala), providing converging evidence on the veracity of these findings, as MEG would be equally sensitive (or insensitive) to such activity across both time points and treatment groups.
Notwithstanding these limitations, the current study provides early neurophysiological evidence of neural alterations following attention training. Our results suggest that observed reductions in PTSD symptomatology may be associated with modulations in disparate cortical and subcortical areas, and future studies should explore the impact of ACT and ABMT using MEG and/or fMRI during cognitive tasks designed to tap specific brain functions (e.g., memory) to further decipher the neurological effects of attention training. The first study of this type found that attention training partially normalized the neurophysiological deficits associated with PTSD during verbal working memory (McDermott et al., 2016), and more such research is certainly warranted as are neurophysiological and imaging studies to examine physiological correlates underlying other therapies for PTSD.
In conclusion, this preliminary study demonstrates that ACT and ABMT appear to modulate a large group of cortical and subcortical brain regions, and that the amplitude of these changes differ in some brain regions based on the type of therapy. Specifically ACT seems to more strongly modulate visual processing pathways, while ABMT appears to affect neural activation in threat processing regions. Both forms of attention training are straightforward, low-cost, low-stress, non-pharmacological interventions, and this study supports the importance of additional research into these emerging therapies for PTSD.
PTSD is associated with neurophysiological aberrations across multiple networks
These activation differences can be partially reversed by attention training
Attention control training modulates visual pathways which are understudied in PTSD
Attention bias modification modulates threat-processing regions in PTSD
Acknowledgments
Grant Support: This research was supported by a grant from the nonprofit organization At Ease, USA (ABB), grant R01-MH103220 from the National Institutes of Health (TWW), NSF grant #1539067, and support from the Intramural Research Program of the National Institute of Mental Health (DSP). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Disclosures: Dr. Badura-Brack, Dr. Heinrichs-Graham, Ms. Becker, Mr. McDermott, Ms. Ryan, and Drs. Khanna, Pine, Bar-Haim, and Wilson report no competing financial or other interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th. Author; Washington, DC: 2013. [Google Scholar]
- Amir N, Beard C, Taylor C, Klumpp H, Elias J, Burns M, et al. Attention training in individuals with generalized social phobia: a randomized controlled trial. J Consult Clin Psychol. 2009;77:961–973. doi: 10.1037/a0016685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aupperle RL, Melrose AJ, Stein MB, Paulus MP. Executive function and PTSD: disengaging from trauma. Neuropharmacology. 2012;62:686–694. doi: 10.1016/j.neuropharm.2011.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badura-Brack AS, Naim R, Ryan TJ, Levy O, Abend R, Khanna MM, et al. Effect of attention training on attention bias variability and PTSD symptoms: randomized controlled trials in Israeli and U.S. combat veterans. Am J Psychiatry. 2015;172:1233–1241. doi: 10.1176/appi.ajp.2015.14121578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badura-Brack AS, Heinrichs-Graham E, McDermott TJ, Becker KM, Ryan TJ, Khanna MM, et al. Resting-state neurophysiological abnormalities in posttraumatic stress disorder: a magnetoencephalography study. Front Hum Neurosci. 2017 doi: 10.3389/fnhum.2017.00205. https://doi.org/10.3389/fnhum.2017.00205. [DOI] [PMC free article] [PubMed]
- Bagby RM, Parker JD, Taylor GJ. The twenty-item Toronto Alexithymia Scale--I. item selection and cross-validation of the factor structure. J Psychosom Res. 1994;38:23–32. doi: 10.1016/0022-3999(94)90005-1. [DOI] [PubMed] [Google Scholar]
- Bagalman E. Mental disorders among OEF/OIF veterans using VA health care: facts and figures. Washington: 2013. Available at: https://fas.org/sgp/crs/misc/R41921.pdf. [Google Scholar]
- Bar-Haim Y. Research review: attention bias modification (ABM): a novel treatment for anxiety disorders. J Child Psychol Psychiatry. 2010;51:859–870. doi: 10.1111/j.1469-7610.2010.02251.x. [DOI] [PubMed] [Google Scholar]
- Bar-Haim Y, Holoshitz Y, Eldar S, Frenkel T, Muller D, Charney D, et al. Life-threatening danger and suppression of attention bias to threat. Am J Psychiatry. 2010;167:694–698. doi: 10.1176/appi.ajp.2009.09070956. [DOI] [PubMed] [Google Scholar]
- Beevers C, Lee HJ, Wells T, Ellis A, Telch M. Association of predeployment gaze bias for emotion stimuli with later symptoms of PTSD and depression in soldiers deployed in Iraq. Am J Psychiatry. 2011;168:735–741. doi: 10.1176/appi.ajp.2011.10091309. [DOI] [PubMed] [Google Scholar]
- Blair KS, Vythilingam M, Crowe SL, McCaffrey DE, Ng P, Wu CC, et al. Cognitive control of attention is differentially affected in trauma-exposed individuals with and without post-traumatic stress disorder. Psychol Med. 2013;43:85–95. doi: 10.1017/S0033291712000840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, et al. The development of a Clinician-Administered PTSD Scale. J Trauma Stress. 1995;8:75–90. doi: 10.1007/BF02105408. [DOI] [PubMed] [Google Scholar]
- Brewin CR. Episodic memory, perceptual memory, and their interaction: foundations for a theory of posttraumatic stress disorder. Psychol Bull. 2014;140:69–97. doi: 10.1037/a0033722. [DOI] [PubMed] [Google Scholar]
- Brewin CR, Burgess N. Contextualisation in the revised dual representation theory of PTSD: a response to Pearson and colleagues. J Behav Ther Exp Psychiatry. 2014;45:217–219. doi: 10.1016/j.jbtep.2013.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewin CR, Dalgleish T, Joseph S. A dual representation theory of posttraumatic stress disorder. Psychol Rev. 1996;103:670–686. doi: 10.1037/0033-295x.103.4.670. [DOI] [PubMed] [Google Scholar]
- Brewin CR, Gregory JD, Lipton M, Burgess N. Intrusive images in psychological disorders: characteristics, neural mechanisms, and treatment implications. Psychol Rev. 2010;117:210–232. doi: 10.1037/a0018113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley TC, Blanchard EB, Neill WT. Information processing and PTSD: a review of the empirical literature. Clin Psychol Rev. 2000;20:1041–1065. doi: 10.1016/s0272-7358(99)00030-6. [DOI] [PubMed] [Google Scholar]
- Cisler JM, Wolitzky-Taylor KB, Adams TG, Jr, Babson KA, Badour CL, et al. The emotional Stroop task and posttraumatic stress disorder: a meta-analysis. Clin Psychol Rev. 2011;31:817–828. doi: 10.1016/j.cpr.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constans JI, McCloskey MS, Vasterling JJ, Brailey K, Mathews A. Suppression of attentional bias in PTSD. J Abnorm Psychol. 2004;113:315–323. doi: 10.1037/0021-843X.113.2.315. [DOI] [PubMed] [Google Scholar]
- Cornwell BR, Arkin N, Overstreet C, Carver FW, Grillon C. Distinct contributions of human hippocampal theta to spatial cognition and anxiety. Hippocampus. 2012;22:1848–1059. doi: 10.1002/hipo.22019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornwell BR, Johnson LL, Holroyd T, Carver FW, Grillon C. Human hippocampal and parahippocampal theta during goal-directed spatial navigation predicts performance on a virtual Morris water maze. J Neurosci. 2008;28:5983–5990. doi: 10.1523/JNEUROSCI.5001-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornwell BR, Overstreet C, Grillon C. Spontaneous fast gamma activity in the septal hippocampal region correlates with spatial learning in humans. Behav Brain Res. 2014;261:258–264. doi: 10.1016/j.bbr.2013.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornwell BR, Salvadore G, Colon-Rosario V, Latov DR, Holroyd T, Carver FW, et al. Abnormal hippocampal functioning and impaired spatial navigation in depressed individuals: evidence from whole-head magnetoencephalography. Am, J Psychiatry. 2010;167:836–44. doi: 10.1176/appi.ajp.2009.09050614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalal SS, Guggisberg AG, Edwards E, Sekihara K, Findlay AM, Canolty RT, et al. Five-dimensional neuroimaging: localization of the time-frequency dynamics of cortical activity. NeuroImage. 2008;40:1686–1700. doi: 10.1016/j.neuroimage.2008.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunkley BT, Doesburg SM, Jetly R, Sedge PA, Pang EW, Taylor MJ. Characterising intra- and inter-intrinsic network synchrony in combat-related post-traumatic stress disorder. Psychiatry Res. 2015;234:172–181. doi: 10.1016/j.pscychresns.2015.09.002. [DOI] [PubMed] [Google Scholar]
- Dunkley BT, Doesburg SM, Sedge PA, Grodecki RJ, Shek PN, Pang EW, et al. Resting-state hippocampal connectivity correlates with symptom severity in post-traumatic stress disorder. Neuroimage Clin. 2014;5:377–384. doi: 10.1016/j.nicl.2014.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunkley BT, Sedge PA, Doesburg SM, Grodecki RJ, Jetly R, Shek PN, et al. Theta, mental flexibility, and post-traumatic stress disorder: connecting the pariental cortex. PLoS ONE. 2015;10:e0123541. doi: 10.1371/journal.pone.0123541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckart C, Stoppel C, Kaufmann J, Tempelmann C, Hinrichs H, Elbert T, et al. Structural alterations in lateral prefrontal, parietal and posterior midline regions of men with chronic posttraumatic stress disorder. J Psychiatry Neurosci. 2011;36:176–186. doi: 10.1503/jpn.100010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers A, Clark DM. A cognitive model of posttraumatic stress disorder. Behav Res Ther. 2000;38:319–345. doi: 10.1016/s0005-7967(99)00123-0. [DOI] [PubMed] [Google Scholar]
- Ehlers A, Hackmann A, Steil R, Clohessy S, Wenninger K, Winter H. The nature of intrusive memories after trauma: the warning signal hypothesis. Behav Res Ther. 2002;40:995–1002. doi: 10.1016/s0005-7967(01)00077-8. [DOI] [PubMed] [Google Scholar]
- Fani N, Tone EB, Phifer J, Norrholm SD, Bradley B, Ressler KJ, et al. Attention bias toward threat is associated with exaggerated fear expression and impaired extinction in PTSD. Psychol Med. 2012;42:533–543. doi: 10.1017/S0033291711001565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foa EB, Gillihan SJ, Bryant RA. Challenges and successes in dissemination of evidence-based treatments for posttraumatic stress: lessons learned from prolonged exposure therapy for PTSD. Psychol Sci Public Interest. 2013;14:65–111. doi: 10.1177/1529100612468841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes D, Creamer M, Bisson J, Cohen J, Crow B, Foa E, et al. A guide to guidelines for the treatment of PTSD and related conditions. J Trauma Stress. 2010;23:537–552. doi: 10.1002/jts.20565. [DOI] [PubMed] [Google Scholar]
- Friston K, Harrison L, Daunizeau J, Kiebel S, Phillips C, Trujillo-Barreto N, et al. Multiple sparse priors for the M/EEG inverse problem. NeuroImage. 2008;39:1104–1120. doi: 10.1016/j.neuroimage.2007.09.048. [DOI] [PubMed] [Google Scholar]
- Gong Q, Li L, Du M, Pettersson-Yeo W, Crossley N, Yang X, Li J, et al. Quantitative prediction of individual psychopathology in trauma survivors using resting-state FMRI. Neuropsychopharmacology. 2014;39:681–687. doi: 10.1038/npp.2013.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackmann A, Ehlers A, Speckens A, Clark D. Characteristics and content of intrusive memories in PTSD and their changes with treatment. J Trauma Stress. 2004;17:231–240. doi: 10.1023/B:JOTS.0000029266.88369.fd. [DOI] [PubMed] [Google Scholar]
- Hakamata Y, Lissek S, Bar-Haim Y, Britton JC, Fox NA, Leibenluft E, et al. Attention bias modification treatment: a meta-analysis toward the establishment of novel treatment for anxiety. Biol Psychiatry. 2010;68:982–990. doi: 10.1016/j.biopsych.2010.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang MXX, Yurgil KA, Robb A, Angeles A, Diwakar M, Risbrough VB, et al. Voxel-wise resting-state MEG source magnitude imaging study reveals neurocircuitry abnormality in active-duty services members and veterans with PTSD. Neuroimage Clin. 2014;5:408–419. doi: 10.1016/j.nicl.2014.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallion LS, Ruscio AM. A meta-analysis of the effect of cognitive bias modification on anxiety and depression. Psychol Bull. 2011;137:940–958. doi: 10.1037/a0024355. [DOI] [PubMed] [Google Scholar]
- Henson RN, Mattout J, Phillips C, Friston KJ. Selecting forward models for MEG source-reconstruction using model-evidence. NeuroImage. 2009;46:168–176. doi: 10.1016/j.neuroimage.2009.01.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacoviello BM, Wu G, Abend R, Murrough JW, Feder A, Fruchter E, et al. Attention bias variability and symptoms of posttraumatic stress disorder. J Trauma Stress. 2014;27:232–239. doi: 10.1002/jts.21899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler K, Biermann-Ruben K, Jonas M, Siebner HR, Bäumer T, Münchau A, et al. Investigating the human mirror neuron system by means of cortical synchronization during the imitation of biological movements. NeuroImage. 2006;33:227–38. doi: 10.1016/j.neuroimage.2006.06.014. [DOI] [PubMed] [Google Scholar]
- Khanna MM, Badura-Brack AS, McDermott TJ, Shepherd A, Ryan TJ, Heinrichs-Graham, et al. Veterans with PTSD engage threat detection but not emotional regulation networks during an emotional Stroop task. Psychol Med. doi: 10.1017/S0033291717000460. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroenke K, Spitzer R, Williams J. The PHQ-9. J Gen Inter Med. 2001;16:606613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuckertz JM, Amir N, Boffa JW, Warren CK, Rindt SE, Norman S. The effectiveness of an attention bias modification program as an adjunctive treatment for post-traumatic stress disorder. Behav Res Ther. 2014;63:25–35. doi: 10.1016/j.brat.2014.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanius RA, Bluhm RL, Coupland NJ, Hegadoren KM, Rowe B, Théberge J. Default mode network connectivity as a predictor of post-traumatic stress disorder symptom severity in acutely traumatized subjects. Acta Psychiatr Scand. 2009;121:33–40. doi: 10.1111/j.1600-0447.2009.01391.x. [DOI] [PubMed] [Google Scholar]
- Leskin LP, White PM. Attentional networks reveal executive function deficits in posttraumatic stress disorder. Neuropsychology. 2007;21:275–284. doi: 10.1037/0894-4105.21.3.275. [DOI] [PubMed] [Google Scholar]
- Liu Y, Li YJJ, Luo EPP, Lu HBB, Yin H. Cortical thinning in patients with recent onset post-traumatic stress disorder after a single prolonged trauma exposure. PLoS ONE. 2012;7:e39025. doi: 10.1371/journal.pone.0039025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López JD, Litvak V, Espinosa JJ, Friston K, Barnes GR. Algorithmic procedures for Bayesian MEG/EEG source reconstruction in SPM. NeuroImage. 2013;84:476–487. doi: 10.1016/j.neuroimage.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott TJ, Badura-Brack AS, Becker KM, Ryan TJ, Khanna MM, Heinrichs-Graham E, et al. Male veterans with PTSD exhibit aberrant neural dynamics during working memory processing. J Psychiatry Neurosci. 2016;41:251–260. doi: 10.1503/jpn.150058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott TJ, Badura-Brack AS, Becker KM, Ryan TJ, Bar-Haim Y, Pine DS, et al. Attention training improves aberrant neural dynamics during working memory processing in veterans with PTSD. Cogn Affect Behav Neurosci. 2016;16:1140–1149. doi: 10.3758/s13415-016-0459-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misic B, Dunkely BT, Sedge PA, Da Costa L, Fatima Z, Berman MG, et al. Post-traumatic stress constrains the dynamic repertoire of neural activity. J Neurosci. 2016;36:419–431. doi: 10.1523/JNEUROSCI.1506-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morey RA, Gold AL, LaBar KS, Beall SK, Brown VM, Haswell CC, et al. Amygdala volume changes in posttraumatic stress disorder in a large case-controlled veterans group. Arch Gen Psychiatry. 2012;69:1169–1178. doi: 10.1001/archgenpsychiatry.2012.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller-Pfeiffer C, Schick M, Schulte-Vels T, O'Gorman R, Michels L, Martin-Soelch C, et al. Atypical visual processing in posttraumatic stress disorder. NeuroImage Clinical. 3:531–538. doi: 10.1016/j.nicl.2013.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthuraman M, Hellriegel H, Hoogenboom N, Anwar AR, Mideksa KG, Krause H, et al. Coherent source and connectivity analysis on simultaneously measured EEG and MEG data during isometric contraction. Conf Proc IEEE Eng Med Biol Soc. 2014;2014:6365–6368. doi: 10.1109/EMBC.2014.6945084. [DOI] [PubMed] [Google Scholar]
- Naim R, Abend R, Wald I, Eldar S, Levi O, Fruchter E, et al. Threat-related attention bias variability and post traumatic stress. Am J Psychiatry. 2015;172:1242–1250. doi: 10.1176/appi.ajp.2015.14121579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olthuis JV, Wozney L, Asmundson GJ, Cramm H, Lingley-Pottie P, McGrath PJ. Distance-delivered interventions for PTSD: a systematic review and meta-analysis. J Anxiety Disord. 2016;44:9–26. doi: 10.1016/j.janxdis.2016.09.010. [DOI] [PubMed] [Google Scholar]
- Pannu Hayes J, Labar KS, Petty CM, McCarthy G, Morey RA. Alterations in the neural circuitry for emotion and attention associated with posttraumatic stress symptomatology. Psychiatry Res. 2009;172:7–15. doi: 10.1016/j.pscychresns.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitman RK, Rasmusson AM, Koenen KC, Shin LM, Orr SP, Gilbertson MW, et al. Biological studies of post-traumatic stress disorder. Nat Rev Neurosci. 2012;13:769–787. doi: 10.1038/nrn3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proskovec AL, Heinrichs-Graham E, Wilson TW. Aging modulates the oscillatory dynamics underlying successful working memory encoding and maintenance. Hum Brain Mapp. 2016;37:2348–2361. doi: 10.1002/hbm.23178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pu Y, Cornwell BR, Cheyne D, Johnson BW. The functional role of human right hippocampal/parahippocampal theta rhythm in environmental encoding during virtual spatial navigation. Hum Brain Mapp. 2017;38:1347–1361. doi: 10.1002/hbm.23458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin LD, Wang Z, Sun YWW, Wan JQQ, Su SSS, Zhou Y, et al. A preliminary study of alterations in default network connectivity in post-traumatic stress disorder patients following recent trauma. Brain Research. 2012;1484:50–56. doi: 10.1016/j.brainres.2012.09.029. [DOI] [PubMed] [Google Scholar]
- Rabinak CA, Angstadt M, Welsh RC, Kenndy AE, Lyubkin M, Martis B, et al. Altered amygdala resting-state functional connectivity in post-traumatic stress disorder. Front Psychiatry. 2011;2:62. doi: 10.3389/fpsyt.2011.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen RC, Ruzek JI, Karlin BE. Evidence-based training in the era of evidence-based practice: challenges and opportunities for training of PTSD providers. Behav Res Ther. 2017;88:37–48. doi: 10.1016/j.brat.2016.07.009. [DOI] [PubMed] [Google Scholar]
- Salvadore G, Cornwell BR, Colon-Rosario V, Coppola R, Grillon C, Zarate CA, et al. Increased anterior cingulate cortical activity in response to fearful faces: a neurophysiological biomarker that predicts rapid antidepressant response to ketamine. Biol Psychiatry. 2009;65:289–295. doi: 10.1016/j.biopsych.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvadore G, Cornwell BR, Sambataro F, Latov D, Colon-Rosario V, Carver F, et al. Anterior cingulate desynchronization and functional connectivity with the amygdala during a working memory task predict rapid antidepressant response to ketamine. Neuropsychopharmacology. 2010;35:1415–1422. doi: 10.1038/npp.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt N, Richey A, Buckner J, Timpano K. Attention training for generalized social anxiety disorder. J Abnorm Psychol. 2009;118:5–14. doi: 10.1037/a0013643. [DOI] [PubMed] [Google Scholar]
- Schoorl M, Putman P, Van Der Does W. Attentional bias modification in posttraumatic stress disorder: a randomized controlled trial. Psychother Psychosom. 2013;82:99–105. doi: 10.1159/000341920. [DOI] [PubMed] [Google Scholar]
- Schuff N, Zhang Y, Zhan W, Lenoci M, Ching C, Boreta L, et al. Patterns of altered cortical perfusion and diminished subcortical integrity in posttraumatic stress disorder: an MRI study. Neuroimage. 2011;54(Suppl 1):S62–8. doi: 10.1016/j.neuroimage.2010.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(Suppl 20):22–33. [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA. Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press; 1983. [Google Scholar]
- Sripada RK, King AP, Garfinkel SN, Wang X, Sripada CS, Welsh RC, et al. Altered resting-state amygdala functional connectivity in men with posttraumatic stress disorder. J Psychiatry Neurosci. 2012;37:241–249. doi: 10.1503/jpn.110069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taulu S, Simola J. Spatiotemporal signal space separation method for rejecting nearby interference in MEG measurements. Phys Med Biol. 2006;51:1759–1768. doi: 10.1088/0031-9155/51/7/008. [DOI] [PubMed] [Google Scholar]
- Todd RM, MacDonald MJ, Sedge P, Robertson A, Jetly R, Taylor MJ, et al. Soliders with posttraumatic stress disorder see a world full of threat: magnetoencephalography reveals enhanced tuning to combat-realted cues. Biol Psychiatry. 2015;78:821–829. doi: 10.1016/j.biopsych.2015.05.011. [DOI] [PubMed] [Google Scholar]
- Wald I, Lubin G, Holoshitz Y, Muller D, Fruchter E, Pine DS, et al. Battlefield-like stress following simulated combat and suppression of attention bias to threat. Psychol Med. 2011;41:699–707. doi: 10.1017/S0033291710002308. [DOI] [PubMed] [Google Scholar]
- Weathers F, Ruscio A, Keane T. Psychometric properties of nine scoring rules for the Clinician-Administered Posttraumatic Stress Disorder Scale. Psychol Assessment. 1999;11:124–133. [Google Scholar]
- Wilson TW, Proskovec AL, Heinrichs-Graham E, O'Neill J, Robertson KR, Fox HS, et al. Aberrant neuronal dynamics during working memory operations in the aging HIV-infected brain. Sci Rep. 2017;7:41568. doi: 10.1038/srep41568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson TW, Slason E, Hernandez OO, Asherin R, Reite ML, Teale PD, et al. Aberrant high-frequency desynchronization of cerebellar cortices in early-onset psychosis. Psychiatry Res. 2009;174:47–56. doi: 10.1016/j.pscychresns.2009.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson TW, Slason E, Asherin R, Kronberg E, Reite ML, Teale PD, et al. An extended motor network generates beta and gamma oscillatory perturbations during development. Brain Cogn. 2010;73:75–84. doi: 10.1016/j.bandc.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson TW, Slason E, Asherin R, Kronberg E, Teale PD, Reite ML, et al. Abnormal gamma and beta MEG activity during finger movements in early-onset psychosis. Dev Neuropsychol. 2011;36:596–613. doi: 10.1080/87565641.2011.555573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt DS, Proctor SP, King DW, King LA, Vasterling JJ. Validation of scales from the Deployment Risk and Resilience Inventory in a sample of Operation Iraqi Freedom veterans. Assessment. 2008;15:391–403. doi: 10.1177/1073191108316030. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Wang Z, Qin LD, Wan JQ, Sun YW, Su SS, et al. Early altered resting-state functional connectivity predicts the severity of post-traumatic stress disorder symptoms in acutely traumatized subjects. PLoS ONE. 2012;7:e46833. doi: 10.1371/journal.pone.0046833. [DOI] [PMC free article] [PubMed] [Google Scholar]


