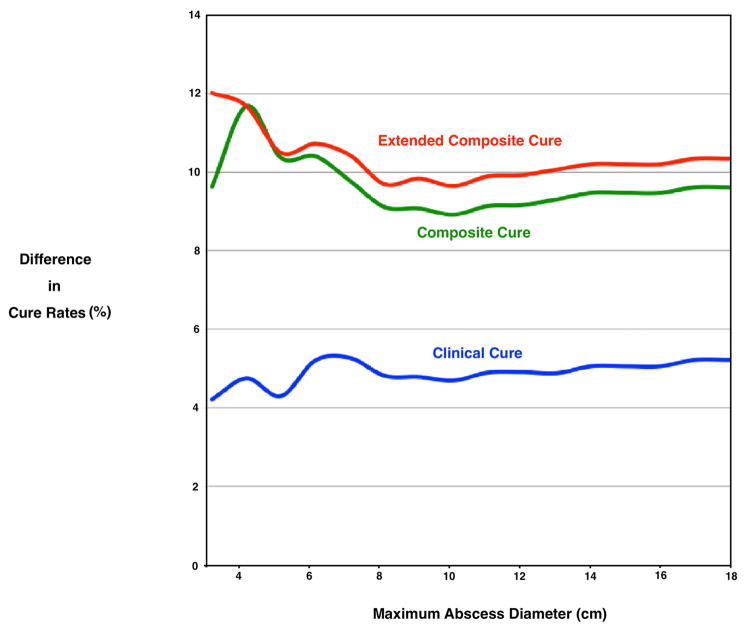
Figure 2.
Difference in Cure Rates* by Abscess Cavity Diameter† Among Participants with a Drained Skin Abscess Treated with Trimethoprim-Sulfamethoxazole Compared to Placebo.
Overall, for trimethoprim-sulfamethoxazole and placebo groups, clinical cure rate at 7–14 days was 92.9% and 85.7% (difference, 7.2; 95% CI 3.2–11.2), composite cure rate at 7–14 was 86.5% and 74.3% (12.2; 95% CI 7.2–17.1) and extended composite cure at 42–56 days was 82.4% and 70.2% (difference, 12.2; 95% CI 6.8–17.6), respectively.
* The between–group percentage point differences in cure rates were plotted by 1 cm increments of abscess cavity or erythema dimension. For each increment, cure rates were calculated for those that had the same or smaller size lesion. Outcomes were defined as follows: clinical cure was defined as resolution of all symptoms and signs of infection, or improvement such that no new antibiotics were prescribed (through 7–14 days after the end of treatment); composite cure was defined as resolution of all symptoms and signs of infection, or improvement such that no additional antibiotics and/or surgical drainage procedure were required (through 7–14 days and 42–56 days after the end of treatment).
†Diameter was defined as the maximal linear dimension (length or width) of the abscess cavity, by probe after incision, and erythema, measured on the skin.