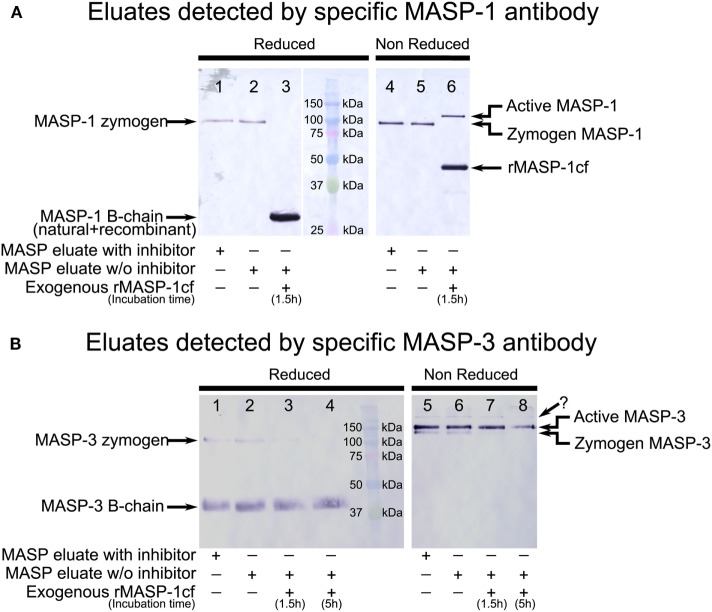
Figure 2.
Western blot detection of mannose-binding lectin (MBL)-associated serine protease (MASP)-1 and MASP-3 isolated from normal human plasma in the presence of inhibitors. A pool of MASPs was purified from human plasma as outlined in Figure 1 in the presence of Pefabloc and 4-nitrophenyl 4′-guanidinobenzoate (NPGB) inhibitors. A sample was prepared in the same manner except that the inhibitors were omitted at the last elution step. Samples were analyzed by SDS-PAGE under reducing and non-reducing conditions followed by Western blotting and detected using a MASP-1- or a MASP-3-specific antibody. Both antibodies were developed against the serine protease (SP) domain of the corresponding protein, hence they can detect the whole molecule, or the B chain of the active form. (A) MASP-1 was present as a zymogen in the isolated samples running at about 95 kDa under reducing conditions. When exogenous active recombinant MASP-1cf was added to the inhibitor-free sample, plasma MASP-1 is converted to the active form. The B chains of plasma MASP-1 and MASP-1cf both ran at about 28 kDa under reducing conditions. Under non-reducing conditions, activation of plasma MASP-1 produced a slower-migrating band compared to the zymogen form. MASP-1cf runs at about 45 kDa under non-reducing conditions. (B) MASP-3 was present both in the zymogen and the active forms in the isolated samples. The zymogen ran at about 100 kDa under reducing conditions and the (glycosylated) B-chain of the active form ran at about 40 kDa. Under non-reducing conditions active MASP-3 migrated slower than the zymogen form. The faint band, indicated by a question mark, running above the active form is probably due to non-specific binding. Addition of active recombinant MASP-1cf to the inhibitor-free sample caused the disappearance of the MASP-3 zymogen bands.