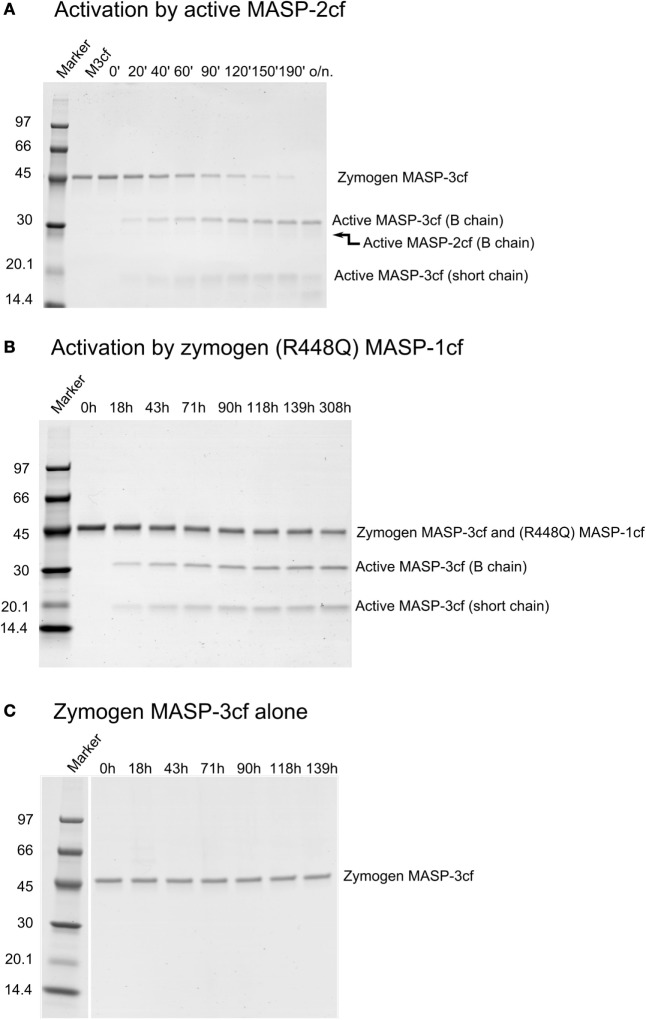
Figure 4.
Activation of zymogen mannose-binding lectin (MBL)-associated serine protease (MASP)-3cf. Zymogen MASP-3cf at 2 µM was incubated with active MASP-2cf, or zymogen R448Q MASP-1cf, or alone. Aliquots were removed periodically at time points as indicated. Samples were analyzed by SDS-PAGE under reducing conditions. Molecular weights of the marker proteins in kDa are indicated. Zymogen MASP-3cf runs at about 48 kDa, while the active form gives two bands at about 31 and 17 kDa. Representative gels are shown as examples. (A). Activation by 91 nM active MASP-2cf. The band corresponding to the B chain of MASP-2cf (about 27 kDa) is very faint due to its low concentration. Lane 2 had zymogen MASP-3cf (M3cf) alone. (B) Activation by 1 µM zymogen R448Q MASP-1cf. The band of zymogen R448Q MASP-1cf (about 46 kDa) comigrated wit that of zymogen MASP-3cf. Quantification was carried out as described in the Section “Materials and Methods” and the determined rate constant are listed in Table 2. (C) Zymogen MASP-3cf alone is shown as a control demonstrating that it does not autoactivate or cleaved by any potential contaminant upon prolonged incubation.