Fig. 6.
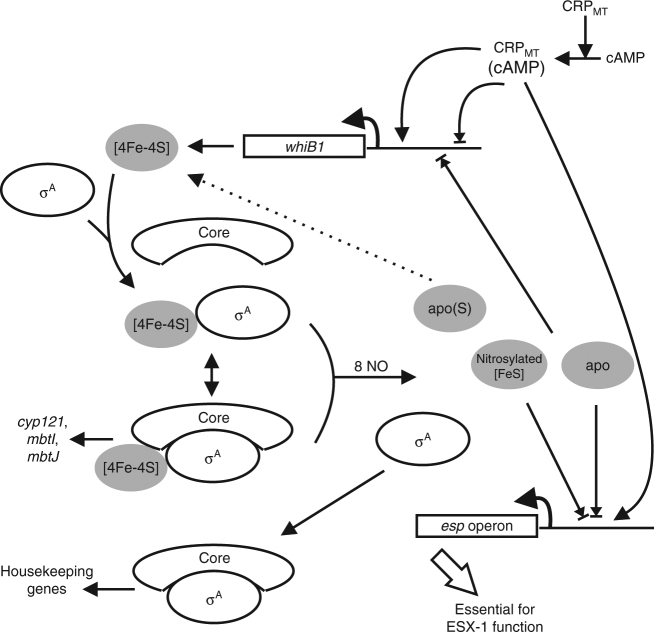
Mycobacterium tuberculosis WhiB1 as a NO-responsive regulator of gene expression. Expression of whiB1 is controlled by the cyclic AMP receptor protein (CRP) in response to cAMP (dual regulation) and by apo-WhiB1 (negative regulation)20,59,60. In the absence of NO, holo-WhiB1 (gray ellipse, [4Fe-4S]) forms a complex with the major sigma factor (open ellipse, σA). The WhiB1:σA complex is likely to be capable of interacting with core RNA polymerase (core) to activate a subset of M. tuberculosis genes, including cyp121, mbtI, and mbtJ, as indicated by the gene expression profiling of cultures underexpressing whiB1 (Table 1). When M. tuberculosis is exposed to NO, the WhiB1 iron–sulfur cluster is nitrosylated, reacting with 8 NO molecules. This results in the liberation of DNA-binding forms of WhiB1 (gray ellipse, apo, and nitrosylated [FeS]) leading to the repression of multiple genes, including the espA operon, which is essential for the function of the virulence-critical ESX-1 secretion system and is also regulated by CRP61. Thus, CRP and WhiB1 combine to integrate inputs from two signaling molecules (cAMP and NO) associated with infection at the esp operon promoter. Intoxication of macrophages by M. tuberculosis derived cAMP is suggested to promote growth and would via the action of the CRP(cAMP) complex activate esp operon expression and ESX-1-mediated secretion62. Precise control of ESX-1 is required because although the secreted effector proteins are essential for infection they are highly antigenic; NO could act as a signal indicating immune system activity resulting in a WhiB1-mediated repression of esp operon expression and shutdown of ESX-1 activity (Supplementary Fig. 13). The retention of sulfur (as persulfides) by WhiB1 offers a route to cluster repair without the need for cysteine desulfurase when NO has been detoxified (dashed line)31