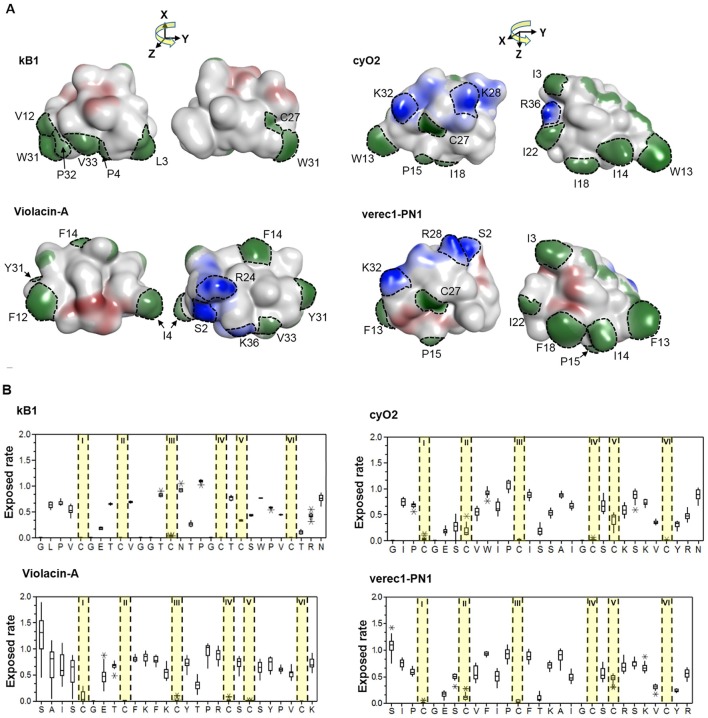
Figure 8.
Membrane-interactive properties of linear and cyclic cyclotides within the Möbius and bracelet lineages. (A) Structures of kalata B1 and violacin A from the Möbius lineage, and cyO2 and verec1-PN1 from the bracelet lineage. Surface-exposed side-chains are shown in blue if positively charged, red if negatively charged, and green if hydrophobic. Notably, the N-terminal residues (S2) of the linear cyclotides always give rise to an exposed positive charge on the molecular surface. The numbering of residues follow the consensus sequence of the cyclotide domain in Figure 3. (B) Exposed ratios of linear and cyclic cyclotides' residues within each lineage. The exposure ratio compares the solvent-exposed surface area (SASA) of each residue in a protein to its SASA in the tripeptide Gly-X-Gly (ψ = ϕ = 180°); the more exposed the residue is, the greater the value. With the exception of the residues in loop 6, residues of cyclic and linear cyclotides have similar exposure ratios at corresponding positions. For each residue, the boxes show the range between the first and third quartiles, and the upper and lower error bars represent the minimum and maximum exposure ratios, respectively.