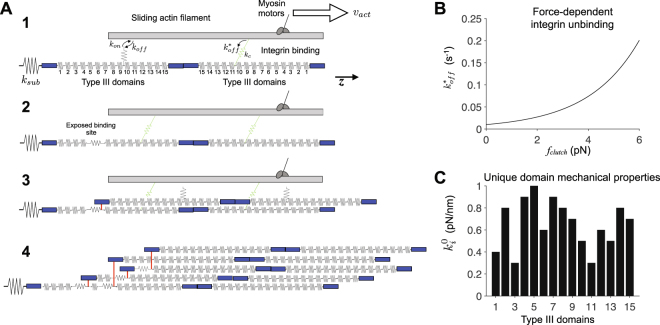
Figure 1.
Diagram and key properties of the fibronectin assembly model. (A) Illustration of fibril assembly. 1. Assembly begins with a single fibronectin (FN) molecule, represented by 30 springs in series, attached to an elastic substrate, with stiffness k sub. Myosin motors pull on the actin filament at velocity v act along the z-axis. Integrins (i.e., molecular clutches) reversibly bind the actin filament with rates k on and k off. Bound integrins transmit a force proportional to the clutch stiffness k c, and unbind with a force-dependent off-rate . Note that integrin springs are connected in parallel with springs representing FN Type III domains. 2. Actomyosin-driven stretch FN Type III domains, exposing a cryptic FN binding site. 3. A soluble FN molecule in the extracellular space binds to the exposed binding site. 4. Subsequent integrin binding, FN Type III domain stretching, and FN-FN binding events produce an elastic, insoluble FN fibril. Adapted from Weinberg et al.40. (B) In contrast with the constant integrin binding on-rate k on, integrin unbinding off-rate increases exponentially as a function of integrin spring or “clutch” force f clutch, given by , where k off is the integrin unbinding rate in the absence of force and f b is a characteristic “break” force. (C) Each Type III domain has a unique characteristic resting stiffness , for i = 1, 2, …, 15.