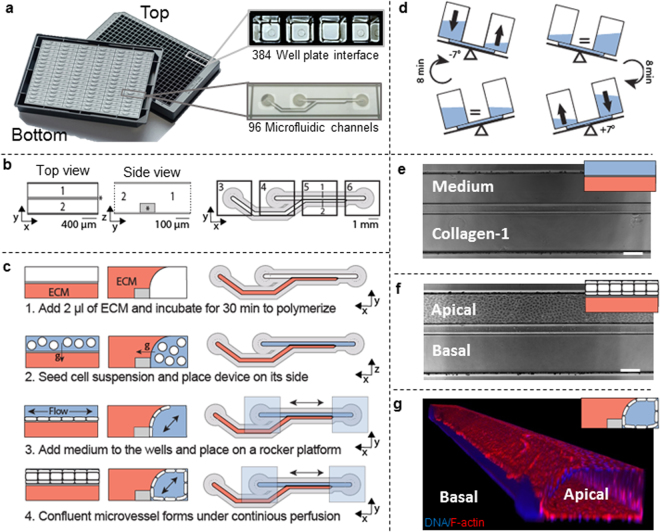
Figure 1.
Microfluidic platform for robust culture of perfusable microvessels. (a) The microfluidic microtiter plate used for perfusable microvessel culture, based on a 384 wells plate interface on top and 96 microfluidic devices integrated in the bottom. (b) Each microfluidic device consists of two channels: an ‘perfusion’ channel (1) and a ‘gel’ channel (2) separated by a phaseguide (*). Every microfluidic structure is positioned underneath 4 adjacent wells. Every first well (3) is positioned on top of the inlet of the gel channel, while every second (4) and fourth well (6) are above respectively the perfusion channel inlet and outlet. Every third well (5) is used for imaging and observation of the experiment. Note that this well does not have an in- or outlet and is therefore not in contact with the microfluidics. Phaseguide, top and bottom substrates are not to scale (c) Method for seeding microvasculature. Collagen-1 gel is seeded as extracellular matrix (ECM) and polymerized (step 1). After polymerization, the cells suspension is seeded in the perfusion channel (step 2). The device is placed on its side to allow the cells the settle and adhere to the collagen-1. After adhesion of the cells, perfusion is started by placing the device on a rocker platform (step 3). In 48 hours the cells grow as a confluent monolayer against the collagen gel and channel walls, resulting in a microvessel with a perfusable lumen (step 4). (d) The rocker platform creates height differences between the wells, which results in a gravity driven, continuous, bi-directional flow. The device is placed at a 7 degree angle which is inverted every 8 minutes. (e) 4× Phase contrast image when imaged below an observation window. Scale bar: 200 µm. (f) 48 hr after cell seeding, a confluent vessel of endothelial cells is formed and an apical side (lumen) and basal side (part of microvessel that adheres to collagen-1) can be distinguished. Scale bar: 200 µm (g) 3D reconstruction of a DAPI/F-actin stained microvessel.