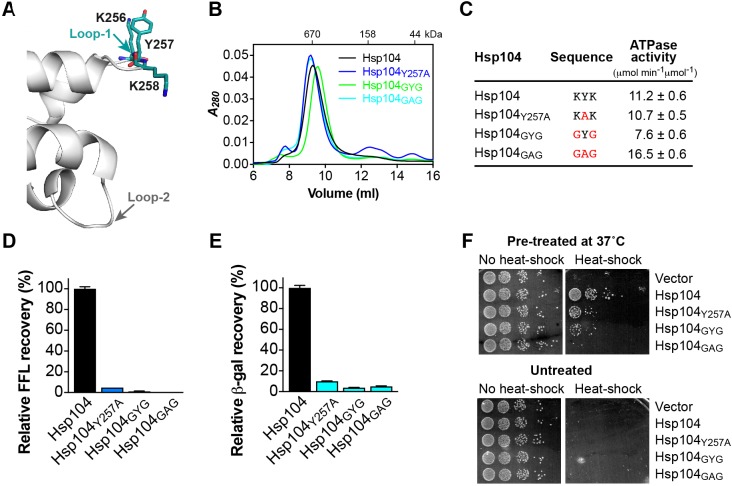
Figure 3. Loop-1 mediates protein–protein interactions essential to Hsp104 function.
(A) Ribbon diagram showing the location of loop-1 relative to loop-2 (gray). Residues of the 256Lys-Tyr-Lys258 tripeptide motif that mediate substrate interaction are colored and shown as stick model. (B) Size-exclusion chromatograms of Hsp104 and loop-1 mutants. (C) ATPase activities of loop-1 mutants. Mutated residues are shown in red. (D,E) Coupled chaperone assay showing the relative recovery of enzymatic activity by loop-1 mutants in the presence of the Hsp70 chaperone system with (D) chemically denatured FFL and (E) heat-aggregated β-gal as substrate. Means of three independent measurements ± S.D. are shown. (F) Induced (top) and basal (bottom) thermotolerance of Δhsp104 yeast expressing the empty vector, Hsp104, or loop-1 mutants.