Fig. 2.
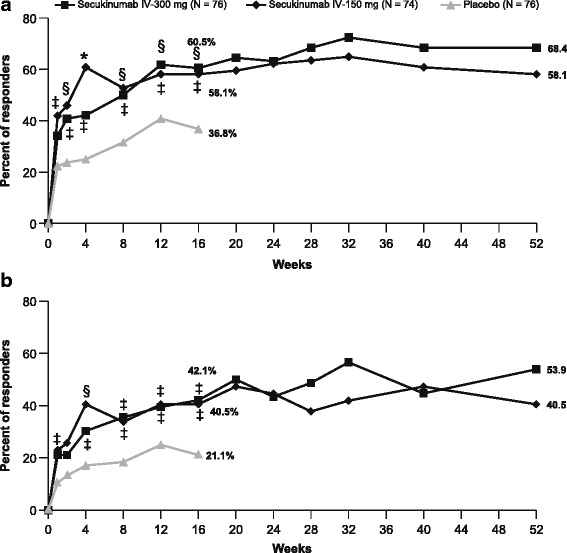
Response rates through week 16 (placebo-controlled phase) and through week 52. Shown are the proportions of patients with Assessment of SpondyloArthritis international Society 20% improvement (ASAS20) responses (improvement of ≥ 20% and absolute improvement of ≥ 1 unit (on a 10-unit scale) in at least three of the four main ASAS domains, with no worsening of ≥ 20% in the remaining domain (a)) and ASAS40 responses (improvement of ≥ 40% and absolute improvement of ≥ 2 units (on a 10-unit scale) in at least three of the four main ASAS domains, with no worsening in the remaining domain (b)). Missing data were imputed as non-responses up to week 52. P values at week 16 were adjusted for multiplicity of testing: * P < 0.0001; § P < 0.01; ‡ P < 0.05 versus placebo
